A. Nekouzadeh, Y. Rudy. Conformational changes of an ion-channel during gating and emerging electrophysiologic properties: Application of a computational approach to cardiac Kv7.1. Prog Biophys Mol Biol. 2016 Jan;120(1-3):18-27. 2015.12.014. Epub 2015 Dec 30. PMCID: PMC4955398.
Nekouzadeh A, Rudy Y. Continuum Molecular Simulation of Large Conformational Changes during Ion-Channel Gating. PLoS One. 2011;6(5):e20186. Epub 2011 May 20.
A modeling framework was developed to simulate large and gradual conformational changes within a macromolecule (protein) when its low amplitude high frequency vibrations are not concerned. Governing equations were derived as alternative to Langevin and Smoluchowski equations and used to simulate gating conformational changes of the Kv7.1 ion-channel over the time scale of its gating process (tens of milliseconds). The alternative equations predict the statistical properties of the motion trajectories with good accuracy and do not require the force field to be constant over the diffusion length, as assumed in Langevin equation. The open probability of the ion channel was determined considering cooperativity of four subunits and solving their concerted transition to the open state analytically. The simulated open probabilities for a series of voltage clamp tests produced current traces that were similar to experimentally recorded currents.
Silva JR, Pan H, Wu D, Nekouzadeh A, Decker KF, Cui J, Baker NA, Sept D, Rudy Y. A multiscale model linking ion-channel molecular dynamics and electrostatics to the cardiac action potential. Proc Natl Acad Sci U S A. 2009 Jul 7;106(27):11102-6. doi: 10.1073/pnas.0904505106. Epub 2009 Jun 22.
Ion-channel function is determined by its gating movement. Yet, molecular dynamics and electrophysiological simulations were never combined to link molecular structure to function. We performed multiscale molecular dynamics and continuum electrostatics calculations to simulate a cardiac K+ channel (IKs) gating and its alteration by mutations that cause arrhythmias and sudden death. An all-atom model of the IKs a-subunit KCNQ1, based on the recent Kv1.2 structure, is used to calculate electrostatic energies during gating. Simulations are compared with experiments where varying degrees of positive charge—added via point mutation—progressively reduce current. Whole-cell simulations show that mutations cause action potential and ECG QT interval prolongation, consistent with clinical phenotypes. This framework allows integration of multiscale observations to study the molecular basis of excitation and its alteration by disease.
Sale H, Wang J, O’Hara TJ, Tester DJ, Phartiyal P, He JQ, Rudy Y, Ackerman MJ, Robertson GA. Physiological properties of hERG 1a/1b heteromeric currents and a hERG 1b-specific mutation associated with Long-QT syndrome. Circ Res. 2008 Sep 26; 103 ( 7 ): e81-95 . Epub 2008 Sep 5.
Cardiac IKr is a critical repolarizing current in the heart and a target for inherited and acquired long-QT syndrome (LQTS). Biochemical and functional studies have demonstrated that IKr channels are heteromers composed of both hERG 1a and 1b subunits, yet our current understanding of IKr functional properties derives primarily from studies of homooligomers of the original hERG 1a isolate. Here, we examine currents produced by hERG 1a and 1a/1b channels expressed in HEK-293 cells at near-physiological temperatures. We find that heteromeric hERG 1a/1b currents are much larger than hERG 1a currents and conduct 80% more charge during an action potential. This surprising difference corresponds to a 2-fold increase in the apparent rates of activation and recovery from inactivation, thus reducing rectification and facilitating current rebound during repolarization. Kinetic modeling shows these gating differences account quantitatively for the differences in current amplitude between the 2 channel types. Drug sensitivity was also different. Compared to homomeric 1a channels, heteromeric 1a/1b channels were inhibited by E-4031 with a slower time course and a corresponding 4-fold shift in the IC50. The importance of hERG 1b in vivo is supported by the identification of a 1b-specific A8V missense mutation in 1/269 unrelated genotype-negative LQTS patients that was absent in 400 control alleles. Mutant 1bA8V expressed alone or with hERG 1a in HEK-293 cells dramatically reduced 1b protein levels. Thus, mutations specifically disrupting hERG 1b function are expected to reduce cardiac IKr and enhance drug sensitivity, and represent a potential mechanism underlying inherited or acquired LQTS.
Nekouzadeh A, Silva JR, Rudy Y. Modeling Subunit Cooperativity in Opening of Tetrameric Ion Channels. Biophys J. 2008 Oct;95(7):3510-20. doi: 10.1529/biophysj.108.136721. Epub 2008 Jul 11.
Most potassium channels are tetramers of four homologous polypeptides (subunits). During channel gating, each subunit undergoes several conformational changes independent of the state of other subunits before reaching a “permissive” state, from which the channel can open. However, transition from the permissive states to the open state involves a concerted movement of all subunits. This cooperative transition must be included in Markov models of channel gating. Previously, it was implemented by considering all possible combinations of four subunit states in a much larger expanded model of channel states (e.g. 27,405 channel states vs. 64 subunit states), which complicates modeling and is computationally intense, especially when accurate modeling requires a large number of subunit states. To overcome these complexities and retain the tetrameric molecular structure, a modeling approach was developed to incorporate the cooperative transition directly from the subunit models. In this approach, the open state is separated from the subunit models and represented by the net flux between the open state and the permissive states. Dynamic variations of the probability of state residencies computed using this direct approach and the expanded model were identical. Implementation of the direct approach is simple and its computational time is orders of magnitude shorter than the equivalent expanded model.
Bébarová M, O’Hara T, Geelen JL, Jongbloed RJ, Timmermans C, Arens YH, Rodriguez LM, Rudy Y, Volders PG. Subepicardial phase 0 block and discontinuous transmural conduction underlie right precordial ST-segment elevation by a SCN5A loss-of-function mutation. Am J Physiol Heart Circ Physiol. 2008 Jul;295(1):H48-58. doi: 10.1152/ajpheart.91495.2007. Epub 2008 May 2.
Two mechanisms are generally proposed to explain right precordial ST-segment elevation in Brugada syndrome: 1) right ventricular (RV) subepicardial action potential shortening and/or loss of dome causing transmural dispersion of repolarization; and 2) RV conduction delay. Here we report novel mechanistic insights into ST-segment elevation associated with a Na+ current (INa) loss-of-function mutation from studies in a Dutch kindred with the COOH-terminal SCN5A variant p.Phe2004Leu. The proband, a man, experienced syncope at age 22 yr and had coved-type ST-segment elevations in ECG leads V1 and V2 and negative T waves in V2. Peak and persistent mutant I(Na) were significantly decreased. INa closed-state inactivation was increased, slow inactivation accelerated, and recovery from inactivation delayed. Computer-simulated INa-dependent excitation was decremental from endo- to epicardium at cycle length 1,000 ms, not at cycle length 300 ms. Propagation was discontinuous across the midmyocardial to epicardial transition region, exhibiting a long local delay due to phase 0 block. Beyond this region, axial excitatory current was provided by phase 2 (dome) of the M-cell action potentials and depended on L-type Ca2+ current (“phase 2 conduction”). These results explain right precordial ST-segment elevation on the basis of RV transmural gradients of membrane potentials during early repolarization caused by discontinuous conduction. The late slow-upstroke action potentials at the subepicardium produce T-wave inversion in the computed ECG waveform, in line with the clinical ECG.
Faber GM, Rudy Y. Calsequestrin mutation and catecholaminergic polymorphic ventricular tachycardia: A simulation study of cellular mechanism. Cardiovasc Res. 2007 Jul 1;75(1):79-88.
OBJECTIVES: Patients with a missense mutation of the calsequestrin 2 gene (CASQ2) are at risk for catecholaminergic polymorphic ventricular tachycardia. In this theoretical study, we investigate a potential mechanism by which CASQ2(D307H) manifests its pro-arrhythmic consequences in patients. METHODS: Using simulations in a model of the guinea pig ventricular myocyte, we investigate the mutation’s effect on SR Ca2+ storage, the Ca2+ transient (CaT), and its indirect effect on ionic currents and membrane potential. We model the effects of isoproterenol (ISO) on Ca(V)1.2 (the L-type Ca2+ current, I(Ca(L))) and other targets of beta-adrenergic stimulation. RESULTS: CASQ2(D307H) reduces SR storage capacity, thereby reducing the magnitude of CaT (Control: 0.79 muM, CASQ2(D307H): 0.52 muM, at cycle length of 1500 ms). The combined effect of CASQ2(D307H) and ISO elevates SR free Ca2+ at a rapid rate, leading to store-overload-induced Ca2+ release and delayed afterdepolarization (DAD). If resting membrane potential is sufficiently elevated, the Na+-Ca2+ exchange-driven DAD can trigger INa and ICa(L) activation, generating a triggered arrhythmogenic AP. CONCLUSIONS: The CASQ2(D307H) mutation manifests its pro-arrhythmic consequences due to store-overload-induced Ca(2+) release and DAD formation due to excess free SR Ca2+ following rapid pacing and beta-adrenergic stimulation.
Faber G, Silva J, Livshitz L, and Rudy Y. Kinetic Properties of the Cardiac L-type Ca Channel and its Role in Myocyte Electrophysiology: A Theoretical Investigation. Biophys. J. published 8 December 2006.
Download: Matlab Simulink code; C++ code
A new, detailed kinetic model of CaV1.2 which is incorporated into a model of the ventricular mycoyte where it interacts with a kinetic model of the ryanodine receptor (RyR) in a restricted subcellular space. We evaluation the contribution of voltagedependent inactivation (VDI) and Ca2+-dependent inactivation (CDI) to total inactivation of CaV1.2. and describe the dynamic CaV1.2 and RyR channel-state occupancy during the AP. Results: 1.) The CaV1.2 model reproduces experimental single-channel and macroscopic-current data. 2.) The model reproduces rate dependence of APD, [Na+]i, and the Ca2+-transient (CaT), and restitution of APD and CaT during premature stimuli. 3.) CDI of CaV1.2 is sensitive to Ca2+ that enters the subspace through the channel and from SR release. The relative contributions of these Ca2+ sources to total CDI during the AP vary with time after depolarization, switching from early SR dominance to late CaV1.2 dominance. 4.) The relative contribution of CDI to total inactivation of CaV1.2 is greater at negative potentials, when VDI is weak. 5.) Loss of VDI due to the CaV1.2 mutation G406R (linked to the Timothy syndrome) results in APD prolongation and increased CaT.
Nekouzadeh A, Rudy Y. Statistical properties of ion channel records. Part II: Estimation from the macroscopic current. Math Biosci. 2007 Nov;210(1):315-34. Epub 2007 May 4.
Macroscopic ion channel current is the summation of the stochastic records of individual channel currents and therefore relates to their statistical properties. As a consequence of this relationship, it may be possible to derive certain statistical properties of single channel records or even generate some estimates of the records themselves from the macroscopic current when the direct measurement of single channel currents is not applicable. We present a procedure for generating the single channel records of an ion channel from its macroscopic current when the stochastic process of channel gating has the following two properties: (I) the open duration is independent of the time of opening event and has a single exponential probability density function (pdf), (II) all the channels have the same probability to open at time t. The application of this procedure is considered for cases where direct measurement of single channel records is difficult or impossible. First, the probability density function (pdf) of opening events, a statistical property of single channel records, is derived from the normalized macroscopic current and mean channel open duration. Second, it is shown that under the conditions (I) and (II), a non-stationary Markov model can represent the stochastic process of channel gating. Third, the non-stationary Markov model is calibrated using the results of the first step. The non-stationary formulation increases the model ability to generate a variety of different single channel records compared to common stationary Markov models. The model is then used to generate single channel records and to obtain other statistical properties of the records. Experimental single channel records of inactivating BK potassium channels are used to evaluate how accurately this procedure reconstructs measured single channel sweeps.
Nekouzadeh A, Rudy Y. Statistical properties of ion channel records. Part I: Relationship to the macroscopic current. Math Biosci. 2007 Nov;210(1):291-314. Epub 2007 May 4.
Macroscopic ion channel current can be derived by summation of the stochastic records of individual channel currents. In this paper, we present two probability density functions of single channel records that can uniquely determine the macroscopic current regardless of other statistical properties of records or the stochastic model of channel gating (presented often with stationary Markov models). We show that H(t), probability density function of channel opening events (introduced explicitly in this paper), and D(t), probability density function of the open duration (sometimes has named dwell time distribution as well), determine the normalized macroscopic current, G(t), throughwhere P(t) is the cumulative density function of H(t), Q(t) is the cumulative density function of D(t), * is the symbol of convolution integral and G(t) is the macroscopic current divided by the amplitude of single channel current and the number of single channel sweeps. Compared to other equations for the macroscopic current, here the macroscopic current is expressed only in terms of the statistical properties of single channel current and not the stochastic model of ion channel gating or a conditioned form of macroscopic current. Single channel currents of an inactivating BK channel were used to validate this relationship experimentally too. In this paper, we used median filters as they can remove the unwanted noise without smoothing the transitions between open and closed states (compare to low pass filters). This filtering leads to more accurate measurement of transition times and less amount of missed events. Click to Enlarge
Click to Enlarge
Clancy CE, Zhu ZI, Rudy Y. Pharmacogenetics and anti-arrhythmic drug therapy: A theoretical investigation. Am J Physiol Heart Circ Physiol. 2006 Sep 22.
We present a theoretical approach for investigating effects of drug-channel interaction. We use as an example open-channel or inactivated-channel block by the local anesthetics mexiletine and lidocaine, respectively, of normal and DKPQ mutant Na+ channels associated with the long-QT syndrome type 3. Results show how kinetic properties of channel gating, which are affected by mutations, are important determinants of drug efficacy. Investigations of Na+ channel blockade are conducted at multiple scales: single channel, macroscopic current and, importantly, during the cardiac action potential (AP). Our findings suggest that channel mean open time is a primary determinant of open state blocker efficacy. The simulations also suggest that inactivation state block by lidocaine is less effective in restoring normal repolarization and adversely suppresses peak Na+ current. Click to Enlarge
Click to Enlarge
Silva, J., Rudy Y. Subunit interaction determines IKs participation in cardiac repolarization and repolarization reserve. Circulation. 2005 Sep 6;112(10):1384-91. Epub 2005 Aug 29.
Download: Matlab code
The role of IKs, the slow delayed rectifier K+ current, in cardiac ventricular repolarization has been a subject of debate. We develop a detailed Markov model of IKs and its ß-subunit KCNQ1 and examine their kinetic properties during the cardiac ventricular action potential at different rates. We observe that interaction between KCNQ1 and KCNE1 (the ß-subunit) confers kinetic properties on IKs that make it suitable for participation in action potential repolarization and its adaptation to rate changes; in particular, the channel develops an available reserve of closed states near the open state that can open rapidly on demand. Because of its ability to form an available reserve, IKs can function as a repolarization reserve when IKr, the rapid delayed rectifier, is reduced by disease or drug and can prevent excessive action potential prolongation and development of arrhythmogenic early afterdepolarizations.
Clancy, CE, Rudy Y. Na(+) channel mutation that causes both Brugada and long-QT syndrome phenotypes: a simulation study of mechanism. Circulation. 2002 Mar 12; 105(10): 1208–1213.
Complex physiological interactions determine the functional consequences of gene abnormalities and make mechanistic interpretation of phenotypes extremely difficult. A recent example is a single mutation in the C terminus of the cardiac Na(+) channel, 1795insD. The mutation causes two distinct clinical syndromes, long QT (LQT) and Brugada, leading to life-threatening cardiac arrhythmias. Coexistence of these syndromes is seemingly paradoxical; LQT is associated with enhanced Na(+) channel function, and Brugada with reduced function. Using a computational approach, we demonstrate that the 1795insD mutation exerts variable effects depending on the myocardial substrate.
Clancy CE, Rudy Y. Cellular consequences of HERG mutations in the long QT syndrome: precursors to sudden cardiac death. Cardiovasc Res. 2001 May;50(2):301-13.
BACKGROUND: A variety of mutations in HERG, the major subunit of the rapidly activating component of the cardiac delayed rectifier I(Kr), have been found to underlie the congenital Long-QT syndrome, LQT2. LQT2 may give rise to severe arrhythmogenic phenotypes leading to sudden cardiac death. OBJECTIVE: We attempt to elucidate the mechanisms by which heterogeneous LQT2 genotypes can lead to prolongation of the action potential duration (APD) and consequently the QT interval on the ECG. METHODS: We develop Markovian models of wild-type (WT) and mutant I(Kr) channels and incorporate these models into a comprehensive model of the cardiac ventricular cell. RESULTS: Using this virtual transgenic cell model, we describe the effects of HERG mutations on the cardiac ventricular action potential (AP) and provide insight into the mechanism by which each defect results in a net loss of repolarizing current and prolongation of APD. CONCLUSIONS: This study demonstrates which mutations can prolong APD sufficiently to generate early afterdepolarizations (EADs), which may trigger life-threatening arrhythmias. The severity of the phenotype is shown to depend on the specific kinetic changes and how they affect I(Kr) during the time course of the action potential. Clarifying how defects in HERG can lead to impaired cellular electrophysiology can improve our understanding of the link between channel structure and cellular function.
Clancy CE, Rudy Y. Linking a genetic defect to its cellular phenotype in a cardiac arrhythmia. Nature. 1999 Aug 5;400(6744):566-9.
Advances in genetics and molecular biology have provided an extensive body of information on the structure and function of the elementary building blocks of living systems. Genetic defects in membrane ion channels can disrupt the delicate balance of dynamic interactions between the ion channels and the cellular environment, leading to altered cell function. As ion-channel defects are typically studied in isolated expression systems, away from the cellular environment where they function physiologically, a connection between molecular findings and the physiology and pathophysiology of the cell is rarely established. Here we describe a single-channel-based Markovian modelling approach that bridges this gap. We achieve this by determining the cellular arrhythmogenic consequences of a mutation in the cardiac sodium channel that can lead to a clinical arrhythmogenic disorder (the long-QT syndrome) and sudden cardiac death.
Decker KF, Heijman J, Silva JR, Hund TJ, Rudy Y. Properties and Ionic Mechanisms of Action Potential Adaptation, Restitution and Accommodation in Canine Epicardium. Am J Physiol Heart Circ Physiol. 2009 Apr;296(4):H1017-26. Epub 2009 Jan 23.
Computational models of cardiac myocytes are important tools for understanding ionic mechanisms of arrhythmia. This work presents a new model of the canine epicardial myocyte that reproduces a wide range of experimentally observed rate dependent behaviors in cardiac cell and tissue, including action potential duration (APD) adaptation, restitution and accommodation. Model behavior depends on updated formulations for the 4-AP sensitive transient outward current (Ito1), the slow component of the delayed rectifier potassium current (IKs), the L-type Ca2+ channel (ICa,L) and the sodium-potassium pump (INaK) fit to data from canine ventricular myocytes. We find that Ito1 plays a limited role in potentiating peak ICa,L and sarcoplasmic reticulum Ca2+ release for propagated APs, but modulates the time course of APD restitution. IKs plays an important role in APD shortening at short diastolic intervals, despite a limited role in AP repolarization at longer cycle lengths. In addition, we find that ICa,L plays a critical role in APD accommodation and rate dependence of APD restitution through its indirect role in intracellular Na+ accumulation and increased outward INaK at rapid heart rates. Our simulation results provide valuable insight into the mechanistic basis of rate-dependent phenomena important for determining the heart’s response to rapid and irregular pacing rates (e.g. arrhythmia). Accurate simulation of rate dependent phenomena and increased understanding of their mechanistic basis will lead to more realistic multicellular simulations of arrhythmia and identification of molecular therapeutic targets.
Rudy Y, Ackerman MJ, Bers DM, Clancy CE, Houser SR, London B, McCulloch AD, Przywara DA, Rasmusson RL, Solaro RJ, Trayanova NA, Van Wagoner DR, Varró A, Weiss JN, Lathrop DA.Systems approach to understanding electromechanical activity in the human heart: a national heart, lung, and blood institute workshop summary. Circulation. 2008 Sep 9;118(11):1202-11.
The National Heart, Lung, and Blood Institute (NHLBI) convened a workshop of cardiologists, cardiac electrophysiologists, cell biophysicists, and computational modelers on August 20 and 21, 2007, in Washington, DC, to advise the NHLBI on new research directions needed to develop integrative approaches to elucidate human cardiac function. The workshop strove to identify limitations in the use of data from nonhuman animal species for elucidation of human electromechanical function/activity and to identify what specific information on ion channel kinetics, calcium handling, and dynamic changes in the intracellular/extracellular milieu is needed from human cardiac tissues to develop more robust computational models of human cardiac electromechanical activity. This article summarizes the workshop discussions and recommendations on the following topics: (1) limitations of animal models and differences from human electrophysiology, (2) modeling ion channel structure/function in the context of whole-cell electrophysiology, (3) excitation-contraction coupling and regulatory pathways, (4) whole-heart simulations of human electromechanical activity, and (5) what human data are currently needed and how to obtain them.
Hund TJ, Decker KF, Kanter E, Mohler PJ, Boyden PA, Schuessler RB, Yamada KA, Rudy Y. Role of activated CaMKII in abnormal calcium homeostasis and I(Na) remodeling after myocardial infarction: Insights from mathematical modeling. J Mol Cell Cardiol.2008 Sep;45(3):420-8. doi: 10.1016/j.yjmcc.2008.06.007. Epub 2008 Jun 28.
Ca2+/calmodulin-dependent protein kinase II is a multifunctional serine/threonine kinase with diverse cardiac roles including regulation of excitation contraction, transcription, and apoptosis. Dynamic regulation of CaMKII activity occurs in cardiac disease and is linked to specific disease phenotypes through its effects on ion channels, transporters, transcription and cell death pathways. Recent mathematical models of the cardiomyocyte have incorporated limited elements of CaMKII signaling to advance our understanding of how CaMKII regulates cardiac contractility and excitability. Given the importance of CaMKII in cardiac disease, it is imperative that computer models evolve to capture the dynamic range of CaMKII activity. In this study, using mathematical modeling combined with biochemical and imaging techniques, we test the hypothesis that CaMKII signaling in the canine infarct border zone (BZ) contributes to impaired calcium homeostasis and electrical remodeling. We report that the level of CaMKII autophosphorylation is significantly increased in the BZ region. Computer simulations using an updated mathematical model of CaMKII signaling reproduce abnormal Ca2+ transients and action potentials characteristic of the BZ. Our simulations show that CaMKII hyperactivity contributes to abnormal Ca2+ homeostasis and reduced action potential upstroke velocity due to effects on I(Na) gating kinetics. In conclusion, we present a new mathematical tool for studying effects of CaMKII signaling on cardiac excitability and contractility over a dynamic range of kinase activities. Our experimental and theoretical findings establish abnormal CaMKII signaling as an important component of remodeling in the canine BZ.
Faber GM, Rudy Y. Calsequestrin mutation and catecholaminergic polymorphic ventricular tachycardia: A simulation study of cellular mechanism. Cardiovasc Res. 2007 Jul 1;75(1):79-88.
OBJECTIVES: Patients with a missense mutation of the calsequestrin 2 gene (CASQ2) are at risk for catecholaminergic polymorphic ventricular tachycardia. In this theoretical study, we investigate a potential mechanism by which CASQ2(D307H) manifests its pro-arrhythmic consequences in patients. METHODS: Using simulations in a model of the guinea pig ventricular myocyte, we investigate the mutation’s effect on SR Ca2+ storage, the Ca2+ transient (CaT), and its indirect effect on ionic currents and membrane potential. We model the effects of isoproterenol (ISO) on Ca(V)1.2 (the L-type Ca2+ current, I(Ca(L))) and other targets of beta-adrenergic stimulation. RESULTS: CASQ2(D307H) reduces SR storage capacity, thereby reducing the magnitude of CaT (Control: 0.79 muM, CASQ2(D307H): 0.52 muM, at cycle length of 1500 ms). The combined effect of CASQ2(D307H) and ISO elevates SR free Ca2+ at a rapid rate, leading to store-overload-induced Ca2+ release and delayed afterdepolarization (DAD). If resting membrane potential is sufficiently elevated, the Na+-Ca2+ exchange-driven DAD can trigger INa and ICa(L) activation, generating a triggered arrhythmogenic AP. CONCLUSIONS: The CASQ2(D307H) mutation manifests its pro-arrhythmic consequences due to store-overload-induced Ca(2+) release and DAD formation due to excess free SR Ca2+ following rapid pacing and beta-adrenergic stimulation.
Faber G, Silva J, Livshitz L, Rudy Y. Kinetic Properties of the Cardiac L-type Ca Channel and its Role in Myocyte Electrophysiology: A Theoretical Investigation. Biophys. J. published 8 December 2006.
Download: Matlab Simulink code; C++ code
A new, detailed kinetic model of CaV1.2 which is incorporated into a model of the ventricular mycoyte where it interacts with a kinetic model of the ryanodine receptor (RyR) in a restricted subcellular space. We evaluation the contribution of voltagedependent inactivation (VDI) and Ca2+-dependent inactivation (CDI) to total inactivation of CaV1.2. and describe the dynamic CaV1.2 and RyR channel-state occupancy during the AP. Results: 1.) The CaV1.2 model reproduces experimental single-channel and macroscopic-current data. 2.) The model reproduces rate dependence of APD, [Na+]i, and the Ca2+-transient (CaT), and restitution of APD and CaT during premature stimuli. 3.) CDI of CaV1.2 is sensitive to Ca2+ that enters the subspace through the channel and from SR release. The relative contributions of these Ca2+ sources to total CDI during the AP vary with time after depolarization, switching from early SR dominance to late CaV1.2 dominance. 4.) The relative contribution of CDI to total inactivation of CaV1.2 is greater at negative potentials, when VDI is weak. 5.) Loss of VDI due to the CaV1.2 mutation G406R (linked to the Timothy syndrome) results in APD prolongation and increased CaT.
Livshitz LM, Rudy Y. Regulation of Ca2+ and electrical alternans in cardiac myocytes: Role of CaMKII and repolarizing currents. Am J Physiol Heart Circ Physiol. 2007 Jun;292(6):H2854-66. Epub 2007 Feb 2.
Download: Matlab code for LRd; Matlab code for HRd
Alternans of cardiac repolarization is associated with arrhythmias and sudden death. At the cellular level, alternans involves beat-to-beat oscillation of the action potential (AP) and possibly Ca2+ transient (CaT). Because of experimental difficulty in independently controlling the Ca2+ and electrical subsystems, mathematical modelling provides additional insights into mechanisms and causality. Pacing protocols were conducted in a canine ventricular myocyte model with the following results: (I) CaT alternans results from refractoriness of the SR Ca2+ release system; alternation of the L-type Ca2+ current (ICa(L)) has a negligible effect; (II) CaT-AP coupling during late AP occurs through the Na+/Ca2+ exchanger (INaCa) and underlies APD alternans; (III) Increased Ca2+/calmodulin-dependent protein kinase II (CaMKII) activity extends the range of CaT and APD alternans to slower frequencies and increases alternans magnitude; its decrease suppresses CaT and APD alternans, exerting an antiarrhythmic effect; (IV). Increase of the rapid delayed rectifier current (IKr) also suppresses APD alternans, but without suppressing CaT alternans. Thus, CaMKII inhibition eliminates APD alternans by eliminating its cause (CaT alternans), while IKr enhancement does so by weakening CaT-APD coupling. The simulations identify combined CaMKII inhibition and IKr enhancement as a possible antiarrhythmic intervention.
Rudy Y, Silva JR. Computational biology in the study of cardiac ion channels and cell electrophysiology. Q Rev Biophys. 2006 Feb;39(1):57-116.
Ion channels are typically studied in isolation (in expression systems or isolated membrane patches), away from the physiological environment of the cell where they interact to generate the AP. A major challenge remains the integration of ion-channel properties into the functioning, complex and highly interactive cell system, with the objective to relate molecular-level processes and their modification by disease to whole-cell function and clinical phenotype. In this article we describe how computational biology can be used to achieve such integration. We explain how mathematical (Markov) models of ion-channel kinetics are incorporated into integrated models of cardiac cells to compute the AP. We provide examples of mathematical (computer) simulations of physiological and pathological phenomena, including AP adaptation to changes in heart rate, genetic mutations in SCN5A and HERG genes that are associated with fatal cardiac arrhythmias, and effects of the CaMKII regulatory pathway and β-adrenergic cascade on the cell electrophysiological function.
Hund TJ, Rudy Y. A role for calcium/calmodulin-dependent protein kinase II in cardiac disease and arrhythmia. Handb Exp Pharmacol. 2006;(171):201-20. Review.
More than 20 years have passed since the discovery that a collection of specific calcium/calmodulin-dependent phosphorylation events is the result of a single multifunctional kinase. Since that time, we have learned a great deal about this multifunctional and ubiquitous kinase, known today as calcium/calmodulin-dependent protein kinase II (CaMKII). CaMKII is interesting not only for its widespread distribution and broad specificity but also for its biophysical properties, most notably its activation by the critical second messenger complex calcium/calmodulin and its autophosphorylating capability. A central role for CaMKII has been identified in regulating a diverse array of fundamental cellular activities. Furthermore, altered CaMKII activity profoundly impacts function in the brain and heart. Recent findings that CaMKII expression in the heart changes during hypertrophy, heart failure, myocardial ischemia, and infarction suggest that CaMKII may be a viable therapeutic target for patients suffering from common forms of heart disease.
Silva J., Rudy Y. Subunit interaction determines IKs participation in cardiac repolarization and repolarization reserve. Circulation. 2005 Sep 6;112(10):1384-91. Epub 2005 Aug 29.
See: IKs Model Details and Matlab Code | IKr Model Details and Matlab Code
The role of IKs, the slow delayed rectifier K+ current, in cardiac ventricular repolarization has been a subject of debate. We develop a detailed Markov model of IKs and its ß-subunit KCNQ1 and examine their kinetic properties during the cardiac ventricular action potential at different rates. We observe that interaction between KCNQ1 and KCNE1 (the ß-subunit) confers kinetic properties on IKs that make it suitable for participation in action potential repolarization and its adaptation to rate changes; in particular, the channel develops an available reserve of closed states near the open state that can open rapidly on demand. Because of its ability to form an available reserve, IKs can function as a repolarization reserve when IKr, the rapid delayed rectifier, is reduced by disease or drug and can prevent excessive action potential prolongation and development of arrhythmogenic early afterdepolarizations.
Hund TJ, Rudy Y. Rate dependence and regulation of action potential and calcium transient in a canine cardiac ventricular cell model. Circulation. 2004 Nov 16;110(20):3168-74.
BACKGROUND: Computational biology is a powerful tool for elucidating arrhythmogenic mechanisms at the cellular level, where complex interactions between ionic processes determine behavior. A novel theoretical model of the canine ventricular epicardial action potential and calcium cycling was developed and used to investigate ionic mechanisms underlying Ca2+ transient (CaT) and action potential duration (APD) rate dependence. METHODS AND RESULTS: The Ca2+/calmodulin-dependent protein kinase (CaMKII) regulatory pathway was integrated into the model, which included a novel Ca2+-release formulation, Ca2+ subspace, dynamic chloride handling, and formulations for major ion currents based on canine ventricular data. Decreasing pacing cycle length from 8000 to 300 ms shortened APD primarily because of I(Ca(L)) reduction, with additional contributions from I(to1), I(NaK), and late I(Na). CaT amplitude increased as cycle length decreased from 8000 to 500 ms. This positive rate-dependent property depended on CaMKII activity. CONCLUSIONS: CaMKII is an important determinant of the rate dependence of CaT but not of APD, which depends on ion-channel kinetics. The model of CaMKII regulation may serve as a paradigm for modeling effects of other regulatory pathways on cell function.
Livshitz L., Rudy Y. Uniqueness and Stability of Action Potential Models during Rest, Pacing, and Conduction Using Problem-Solving Environment. Biophys J. 2009 Sep 2; 97(5): 1265–1276.
Download: Matlab code
Development and application of physiologically detailed dynamic models of the action potential (AP) and Ca 2+ cycling in cardiac cells is a rapidly growing aspect of computational cardiac electrophysiology. Given the large scale of the nonlinear system involved, questions were recently raised regarding reproducibility, numerical stability, and uniqueness of model solutions, as well as ability of the model to simulate AP propagation in multicellular configurations. To address these issues, we reexamined ventricular models of myocyte AP developed in our laboratory with the following results. 1), Recognizing that the model involves a system of differential-algebraic equations, a procedure is developed for estimating consistent initial conditions that insure uniqueness and stability of the solution. 2), Model parameters that can be used to modify these initial conditions according to experimental values are identified. 3), A convergence criterion for steady-state solution is defined based on tracking the incremental contribution of each ion species to the membrane voltage. 4), Singularities in state variable formulations are removed analytically. 5), A biphasic current stimulus is implemented to completely eliminate stimulus artifact during long-term pacing over a broad range of frequencies. 6), Using the AP computed based on 1–5 above, an efficient scheme is developed for computing propagation in multicellular models.
Decker KF, Heijman J, Silva JR, Hund TJ, Rudy Y. Properties and Ionic Mechanisms of Action Potential Adaptation, Restitution and Accommodation in Canine Epicardium. Am J Physiol Heart Circ Physiol . 2009 Apr;296(4):H1017-26. Epub 2009 Jan 23.
Computational models of cardiac myocytes are important tools for understanding ionic mechanisms of arrhythmia. This work presents a new model of the canine epicardial myocyte that reproduces a wide range of experimentally observed rate dependent behaviors in cardiac cell and tissue, including action potential duration (APD) adaptation, restitution and accommodation. Model behavior depends on updated formulations for the 4-AP sensitive transient outward current (Ito1), the slow component of the delayed rectifier potassium current (IKs), the L-type Ca2+ channel (ICa,L) and the sodium-potassium pump (INaK) fit to data from canine ventricular myocytes. We find that Ito1 plays a limited role in potentiating peak ICa,L and sarcoplasmic reticulum Ca2+ release for propagated APs, but modulates the time course of APD restitution. IKs plays an important role in APD shortening at short diastolic intervals, despite a limited role in AP repolarization at longer cycle lengths. In addition, we find that ICa,L plays a critical role in APD accommodation and rate dependence of APD restitution through its indirect role in intracellular Na+ accumulation and increased outward INaK at rapid heart rates. Our simulation results provide valuable insight into the mechanistic basis of rate-dependent phenomena important for determining the heart’s response to rapid and irregular pacing rates (e.g. arrhythmia). Accurate simulation of rate dependent phenomena and increased understanding of their mechanistic basis will lead to more realistic multicellular simulations of arrhythmia and identification of molecular therapeutic targets.
Kleber AG, Rudy Y. Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol Rev84: 431– 488, 2004; 10.1152/physrev.00025.2003.
Propagation of excitation in the heart involves action potential (AP) generation by cardiac cells and its propagation in the multicellular tissue. AP conduction is the outcome of complex interactions between cellular electrical activity, electrical cell-to-cell communication, and the cardiac tissue structure. As shown in this review, strong interactions occur among these determinants of electrical impulse propagation. This review attempts to synthesize results from computer simulations and experimental preparations to define mechanisms and biophysical principles that govern normal and abnormal conduction in the heart.
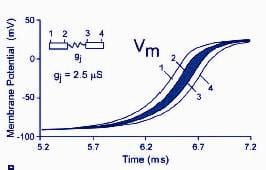
Gima K., Rudy Y. Ionic current basis of electrocardiographic waveforms: a model study. Circ Res 90(8): 889-96.
Body surface electrocardiograms and electrograms recorded from the surfaces of the heart are the basis for diagnosis and treatment of cardiac electrophysiological disorders and arrhythmias. Given recent advances in understanding the molecular mechanisms of arrhythmia, it is important to relate these electrocardiographic waveforms to cellular electrophysiological processes. This modeling study establishes the principles that provide a mechanistic cellular basis for interpretation of electrocardiographic waveforms.
Kucera JP., Rohr S., et al. Localization of sodium channels in intercalated disks modulates cardiac conduction. Circ Res. 2002 Dec 13;91(12):1176-82.
It is well known that the sodium current (INa) and the degree of gap-junctional electrical coupling are the key determinants of action potential (AP) conduction in cardiac tissue. Immunohistochemical studies have shown that sodium channels (NaChs) are preferentially located in intercalated disks (IDs). Using dual immunocytochemical staining, we confirmed the colocalization of NaChs with connexin43 in cultures of neonatal rat ventricular myocytes. In mathematical simulations of conduction using the Luo-Rudy dynamic model of the ventricular AP, we assessed the hypothesis that conduction could be modulated by the preferential localization of NaChs in IDs.
Kucera JP., Rudy Y. Mechanistic insights into very slow conduction in branching cardiac tissue: a model study. Circ Res. 2001 Oct 26;89(9):799-806.
It is known that branching strands of cardiac tissue can form a substrate for very slow conduction. The branches slow conduction by acting as current loads drawing depolarizing current from the main strand (“pull” effect). It has been suggested that, upon depolarization of the branches, they become current sources reinjecting current back into the strand, thus enhancing propagation safety (“push” effect). It was the aim of this study to verify this hypothesis and to assess the contribution of the push effect to propagation velocity and safety.
Wang Y., Rudy Y. Action potential propagation in inhomogeneous cardiac tissue: safety factor considerations and ionic mechanism. Am J Physiol Heart Circ Physiol. 2000 Apr;278(4):H1019-29.
Heterogeneity of myocardial structure and membrane excitability is accentuated by pathology and remodeling. In this study, a detailed model of the ventricular myocyte in a multicellular fiber was used to compute a location-dependent quantitative measure of conduction (safety factor, SF) and to determine the kinetics and contribution of sodium current (INa) and L-type calcium current (ICa(L)) during conduction.
 Click to Enlarge
Click to Enlarge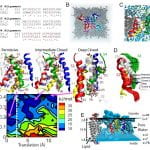 Click to Enlarge
Click to Enlarge Click to Enlarge
Click to Enlarge Click to Enlarge
Click to Enlarge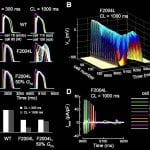 Click to Enlarge
Click to Enlarge Click to Enlarge
Click to Enlarge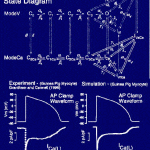 Click to Enlarge
Click to Enlarge Click to Enlarge
Click to Enlarge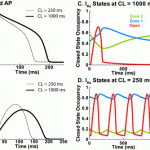 Click to Enlarge
Click to Enlarge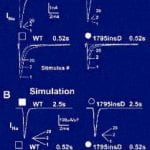 Click to Enlarge
Click to Enlarge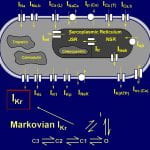 Click to Enlarge
Click to Enlarge Click to Enlarge
Click to Enlarge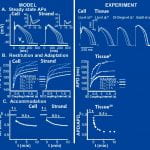 Click to Enlarge
Click to Enlarge Click to Enlarge
Click to Enlarge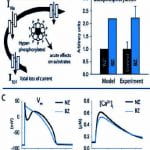 Click to Enlarge
Click to Enlarge Click to Enlarge
Click to Enlarge Click to Enlarge
Click to Enlarge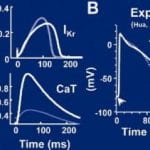 Click to Enlarge
Click to Enlarge Click to Enlarge
Click to Enlarge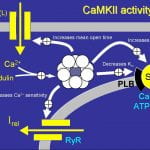 Click to Enlarge
Click to Enlarge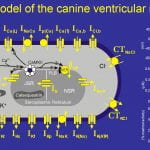 Click to Enlarge
Click to Enlarge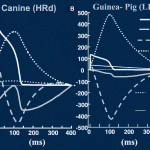 Click to Enlarge
Click to Enlarge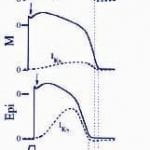 Click to Enlarge
Click to Enlarge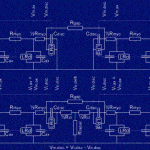 Click to Enlarge
Click to Enlarge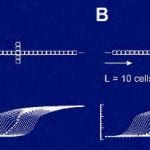 Click to Enlarge
Click to Enlarge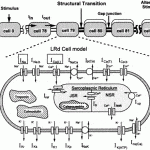 Click to Enlarge
Click to Enlarge