Publication Years: 2002-2004 | 1997-1999 | 1989-1992
2002-2004
C. Ramanathan, R.N. Ghanem, P. Jia, K. Ryu, Y. Rudy, “Electrocardiographic Imaging (ECGI): A Noninvasive Imaging Modality for Cardiac Electrophysiology and Arrhythmia” Nature Medicine 2004; 10:422-428.
Over 7 million people worldwide die annually from erratic heart rhythms (cardiac arrhythmias), and many more are disabled. Yet there is no imaging modality to identify patients at risk, provide accurate diagnosis and guide therapy. Standard diagnostic techniques such as the electrocardiogram (ECG) provide only low-resolution projections of cardiac electrical activity on the body surface. Here we demonstrate the successful application in humans of a new imaging modality called electrocardiographic imaging (ECGI), which noninvasively images cardiac electrical activity in the heart. In ECGI, a multielectrode vest records 224 body-surface electrocardiograms; electrical potentials, electrograms and isochrones are then reconstructed on the heart’s surface using geometrical information from computed tomography (CT) and a mathematical algorithm. We provide examples of ECGI application during atrial and ventricular activation and ventricular repolarization in (i) normal heart (ii) heart with a conduction disorder (right bundle branch block) (iii) focal activation initiated by right or left ventricular pacing, and (iv) atrial flutter.
Y. Rudy , J.E. Burnes, “Noninvasive Electrocardiographic Imaging (ECGI)” Annals of Noninvasive Electrocardiology 1999;4:340-359.
Y. Rudy and H.S. Oster, “The Electrocardiographic Inverse Problem” CRC Critical Reviews in Biomedical Engineering 1992; 20: 25-46.
Publication Years: 2000-2001 | 1989-1992 | 1978-1988
C. Ramanathan, Y. Rudy , “Electrocardiographic Imaging: I. Effect of Torso Inhomogeneities on Body Surface Electrocardiographic Potentials” J Cardiovasc Electrophysiol 2001; 12:229-240
Body surface potential maps (BSPMs) and conventional ECG reflects electrical sources generated by cardiac excitation and repolarization and noninvasively provide important diagnostic information about the electrical state of the heart. Because the heart is located within the torso volume conductor, body surface potentials also reflect the effects of torso inhomogeneities, which include blood, lungs, bone, muscle, fat, and fluid. It is necessary to characterize and understand these effects in order to interpret BSPM and ECG in terms of cardiac activity without “contamination” from the inhomogeneous volume conductor. Actual measured epicardial and body surface potentials were obtained during normal sinus rhythm and for different pacing protocols from a Langendorff-perfused dog heart suspended in a human-shaped torso tank. Accurate geometry of the torso inhomogeneities was digitized from the Visual Human Project and appropriately introduced into a computer model of the tank setup. The geometry and electrical properties of the volume conductor could be varied. Both homogeneous and inhomogeneous torsos have major smoothing effects on BSPM, which is of very low resolution compared with its corresponding epicardial potential pattern. Relative to a homogeneous torso, the inhomogeneities have only a minor effect on BSPM patterns. They augment potential magnitudes depending on the pattern of epicardial activation. Variations of geometry and electrical properties within the normal physiologic range have minimal effects. Effects of torso inhomogeneities on 12-lead ECGs are minimal, and the associated ECG changes fall within the range of normal interindividual variations.
C. Ramanathan, Y. Rudy , “Electrocardiographic Imaging: II. Effect of Torso Inhomogeneities on the Noninvasive Reconstruction of Epicardial Potentials, Electrograms and Isochrones” J Cardiovasc Electrophysiol 2001; 12:241-252.
Noninvasive electrocardiographic imaging (ECGI) involves inverse reconstruction of epicardial potentials, electrograms (EGMs), and isochrones from body surface potential maps (BSPMs). The heart lies in a volume conductor that includes lungs, blood, bone, muscle, and fluid. We investigate the effects of these torso inhomogeneities on reconstructed epicardial potentials, EGMs, and isochrones to address the issue of whether they should be included in clinical ECGI methodology. Potential data were obtained for different pacing protocols from a dog heart suspended in a human-shaped torso tank. Accurate geometry of torso inhomogeneities was digitized from the Visual Human Project and appropriately introduced into a computer model of the torso. Three models were used: accurate inhomogeneous torso, homogeneous torso, and a torso with stylized lungs (to generate an approximate model). The inhomogeneous model was used to compute BSPMs from the measured epicardial potentials. These BSPMs were the starting point for inverse computations in the different torso models. Epicardial potential maps, EGMs, and isochrones were computed. The homogeneous model produced slightly less accurate epicardial potential reconstructions than the inhomogeneous model and stylized lung model, but epicardial potential patterns, EGMs, isochrones, and locations of pacing sites were reconstructed with comparable accuracy when torso inhomogeneities were ignored. The results demonstrate that, in the clinical application, it is not necessary to include torso inhomogeneities for noninvasive reconstructions of epicardial potentials, EGMs, and activation sequences.
D. Khoury and Y. Rudy , “A Model Study of Volume Conductor Effects on Endocardial and Intracavitary Potentials” Circulation Research 1992; 71: 511-525.
B.J.Messinger-Rapport and Y. Rudy , “Computational Issues of Importance to the Inverse Recovery of Epicardial Potential in a Realistic Heart-Torso Geometry” Mathematical Biosciences 1989; 97: 85-120.
Y. Rudy and B.J. Messinger-Rapport, “The Inverse Problem in Electrocardiography: Solutions in terms of Epicardial Potentials” CRC Critical Reviews in Biomedical Engineering 1988; 16: 215-268.
J.N. Amoore and Y. Rudy , “The Effect of Variations of Ventricular Volume on the Electrocardiogram. A Comparison of Two Models” J Electrocardiology 1988; 21: 154-160.
B.J. Messinger-Rapport and Y. Rudy , “The Inverse Problem in Electrocardiography: A Model Study of the Effects of Geometry and Conductivity Parameters on the Reconstruction of Epicardial Potentials” IEEE Trans Biomed Eng BME 1986; 33: 667-676.
B.J. Messinger-Rapport and Y. Rudy , “Effects of the Torso Boundary and Internal Conductivity Interfaces in Electrocardiography: An Evaluation of the ‘Infinite Medium’ Approximation” Bulletin of Mathematical Biology 1985; 47: 685-694.
Publication Years: 2005-2006 | 2002-2004 | 1997-1999 | 1989-1992 | 1978-1988
2005-2006
Y. Wang, Y. Rudy, “Application of the Method of Fundamental Solutions to Potential-based Inverse Electrocardiography” Annals of Biomedical Engineering 2006; 34:1272-1288.
The method of choice for computing epicardial potentials in ECGI has been the Boundary Element Method (BEM) which requires meshing the heart and torso surfaces and optimizing the mesh, a very time-consuming operation that requires manual editing. Moreover, it can introduce mesh-related artifacts in the reconstructed epicardial images. Here we introduce the application of a meshless method, the Method of Fundamental Solutions (MFS) to ECGI. This new approach that does not require meshing is evaluated on data from animal experiments and human studies, and compared to BEM. Results demonstrate similar accuracy, with the following advantages: 1. Elimination of meshing and manual mesh optimization processes, thereby enhancing automation and speeding the ECGI procedure. 2. Elimination of mesh-induced artifacts. 3. Elimination of complex singular integrals that must be carefully computed in BEM. 4. Simpler implementation. These properties of MFS enhance the practical application of ECGI as a clinical diagnostic tool.
S. Ghosh,Y. Rudy, “Accuracy of Quadratic versus Linear Interpolation in Noninvasive Electrocardiographic Imaging (ECGI)” Annals of Biomed Eng 2005;33:1187-1201
The ECGI procedure involves solving Laplace’s equation in the source-free volume conductor between torso and epicardial surfaces, using Boundary Element Method (BEM). Previously, linear interpolation (LI) on three-noded triangular surface elements was used in the BEM formulation. Here, we use quadratic interpolation (QI) for potentials over six-noded linear triangles. The performance of LI and QI in ECGI is evaluated through direct comparison with measured data from an isolated canine heart suspended in a human-torso-shaped electrolyte tank. QI enhances the accuracy and resolution of ECGI reconstructions for two different inverse methods, Tikhonov regularization and Generalized Minimal Residual (GMRes) method, with the QI-GMRes combination providing the highest accuracy and resolution. QI reduces the average relative error (RE) between reconstructed and measured epicardial potentials by 25%. It preserves the amplitude (average RE reduced by 48%) and morphology of electrograms better (average correlation coefficient for QI ∼ 0.97, LI ∼ 0.92). We also applied QI to ECGI reconstructions in human subjects during cardiac pacing, where QI locates ventricular pacing sites with higher accuracy (≤10 mm) than LI (≤18 mm).
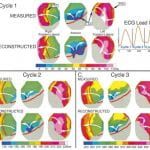
C. Ramanathan, P. Jia, R.N. Ghanem, D. Calvetti, Y. Rudy , “Noninvasive Electrocardiographic Imaging (ECGI): Application of the Generalized Minimal Residual (GMRes) Method“ Annals of Biomedical Engineering 2003; 31:981-994
Electrocardiographic imaging ~ECGI! is a developing imaging modality for cardiac electrophysiology and arrhythmias. It reconstructs epicardial potentials, electrograms, and isochrones from electrocardiographic body-surface potentials noninvasively. Current ECGI methodology employs Tikhonov regularization, which imposes constraints on the reconstructed potentials or their derivatives. This approach can sometimes reduce spatial resolution by smoothing the solution. Accuracy depends on a priori knowledge of solution characteristics and determination of an optimal regularization parameter. These properties led us to implement an independent, iterative approach for ECGI—the generalized minimal residual (GMRes) method—which does not apply constraints. GMRes was applied to experimental data during activation/repolarization of normal and infarcted hearts. GMRes reconstructions were compared to Tikhonov reconstructions and to measured ‘‘gold standards’’ in isolated hearts. Overall, the accuracy of GMRes solutions was similar to Tikhonov regularization. However, in certain cases GMRes recovered localized potential features ~e.g., multiple potential minima!, which were lost in the Tikhonov solution. Simultaneous use of these two complementary methods in clinical ECGI will ensure reliability and maximal extraction of diagnostic information in the absence of a priori information about a patient’s condition.
J.E. Burnes, D.C. Kaelber, B. Taccardi, R.L. Lux, P.R. Ershler, Y. Rudy ,” A Field- Compatible Method for Interpolating Biopotentials: Development and Evaluation in Electrocardiographic Imaging” Annals of Biomedical Engineering 1998; 26: 37-47.
H.S. Oster and Y. Rudy , “Regional Regularization of the Electrocardiographic Inverse Problem: A Model Study using Spherical Geometry” IEEE Trans BME 1997; 44: 188-199.
H.S. Oster and Y. Rudy , “The Use of Temporal Information in the Regularization of the Inverse Problem of Electrocardiography” IEEE Trans Biomed Eng 1992; 39: 65-75.
B.J. Messinger-Rapport and Y. Rudy , “Regularization of the Inverse Problem in Electrocardiography: A Model Study” Mathematical Biosciences 1988; 89: 79-118.
Publication Years: 2002-2004 | 2000-2001 | 1997-1999 | 1993-1996 | 1989-1992
2002-2004
P. Jia, B.Punske, B.Taccardi, Y. Rudy, “Endocardial Mapping of Electrophysiologically Abnormal Substrates and Cardiac Arrhythmias using a Noncontact Nonexpandable Catheter“ J Cardiovasc Electrophysiol 2002;13:888-895
In previous studies, we established methodology for reconstructing endocardial potential maps, electrograms, and isochrones from a noncontact intracavitary catheter during a single beat. Recently, we evaluated this approach using a 9-French (3-mm) spiral catheter in a normal heart preparation. Here we extend the approach to hearts with structural disease and examine its ability to detect and characterize abnormal electrophysiologic (EP) substrates and to map ventricular arrhythmias on a beat-by-beat basis. Reconstruction of endocardial potentials from cavity potentials measured with 82 electrodes mounted on a 9-French spiral catheter was performed in an isolated canine left ventricle (LV). Endocardial potentials were recorded with 91 intramural needles, providing a gold standard for evaluating the noncontact reconstruction. Studies were performed in a normal LV (control) and the same LV 3 hours after left anterior descending coronary artery occlusion and ethanol injection to create an infarct. Abnormal EP characteristics over the infarct were faithfully reconstructed, including (1) low potentials and electrogram derivatives; (2) fractionated electrograms; (3) small deflections on electrograms reflecting local activation; and (4) slow discontinuous conduction transverse to fibers. During arrhythmia, beat-to-beat dynamic shifts of initiation site and activation pattern were captured by the reconstruction. Noncontact, nonexpendable catheter mapping can locate and characterize abnormal EP substrates and can capture the endocardial sequence of an arrhythmia during a single beat.
J.E. Burnes, R.N. Ghanem, A.L. Waldo, Y. Rudy, “Imaging Dispersion of Myocardial Repolarization I. Comparison of Body Surface and Epicardial Measures” Circulation 2001;104:1299 – 1305
Body-surface ECG measures (QT dispersion [QTd], QRST integrals) have been used as indices of myocardial repolarization abnormalities with the goal of identifying patients at risk of fatal arrhythmias. The clinical utility of these measures has been questioned. We investigate the complex relationship between epicardial and body-surface potentials in the context of regionally abnormal myocardial repolarization. Epicardial potentials were recorded with a 224-electrode sock from an open-chest dog during control, regional epicardial warming, cooling, and adjacent warming and cooling to induce localized alterations in myocardial repolarization and regions of increased repolarization dispersion. Body-surface potentials were generated from these epicardial potentials in a human torso model. Epicardial estimates of repolarization (activation recovery intervals [ARIs] and QRST integrals) were evaluated for their ability to identify regions with increased repolarization dispersion. Body-surface QRST integrals and QTd in 12-lead ECG and 64-lead body-surface potential maps were evaluated for their ability to detect increased dispersion of myocardial repolarization. Epicardial ARI and QRST integral maps successfully located epicardial regions with increased dispersion of repolarization. The increased dispersion was not consistently reflected in the 12-lead or 64-lead ECG QTd or in the body-surface QRST integral maps. This study demonstrates the inadequacy of body-surface measures that are thought to reflect myocardial dispersion of repolarization. In contrast, measures based on epicardial electrograms (ARI or epicardial QRST integral maps) provide physiologically relevant information about myocardial repolarization and can locate regions of increased dispersion.
R.N. Ghanem, J.E. Burnes A.L. Waldo, Y. Rudy, “Imaging Dispersion of Myocardial Repolarization II. Noninvasive Reconstruction of Epicardial Measures” Circulation 2001;104:1306 – 1312
Dispersion of myocardial repolarization supports the development and maintenance of life-threatening arrhythmias. Current noninvasive approaches for detecting substrates with increased dispersion based on ECG measures (eg, QT dispersion) have shown limited success and inconsistencies. The companion article shows that, in contrast, epicardial potentials and derived measures reflect local dispersion of repolarization. Here, using a recently developed ECG imaging method, we evaluate the feasibility of noninvasive reconstruction of such epicardial measures from body-surface ECG data. Epicardial potentials were recorded with a 224-electrode sock from an open-chest dog during control, regional warming, cooling, and simultaneous adjacent warming and cooling to induce localized changes in myocardial repolarization and regions of increased dispersion. Body-surface potentials were generated from these epicardial potentials in a human torso model. Realistic geometric errors and measurement noise were added to the torso data, which were then used to noninvasively reconstruct epicardial measures of repolarization dispersion (activation recovery intervals [ARIs] and QRST integrals). Repolarization properties were accurately depicted by ECG imaging, including (1) shortened ARIs and increased QRST integrals over the warmed region, (2) prolonged ARIs and decreased QRST integrals over the cooled region, and (3) high gradients of ARIs and QRST integrals over the adjacent warmed and cooled regions. ECG imaging can reconstruct repolarization properties accurately and localize areas of increased dispersion of repolarization in the heart noninvasively. Its clinical significance lies in the possibility of noninvasive risk stratification and in guidance and evaluation of therapy.
J. E. Burnes, B. Taccardi, P. Ershler, Y. Rudy, “Noninvasive ECG Imaging of Substrate and Intramural Ventricular Tachycardia in Infarcted Hearts” J Amer College Cardiol (JACC) 2001;38:2071-2078.
Myocardial infarction and subsequent remodeling produce abnormal electrophysiologic substrates capable of initiating and maintaining reentrant arrhythmias. Existing noninvasive electrocardiographic methods cannot characterize abnormal electrophysiologic substrates in the heart or the details of associated arrhythmias. A noninvasive method with such capabilities is needed to identify patients at risk of arrhythmias and to guide and evaluate therapy. A dog heart with a four-day-old infarction was suspended in a human shaped torso-tank. Measured body surface potentials were used to noninvasively compute epicardial potentials, electrograms and isochrones. Accuracy of reconstruction was evaluated by direct comparison to measured data. Reconstructions were performed during right atrial pacing and nine cycles of VT. Noninvasively reconstructed potential maps, electrograms and isochrones identified: 1) the location of electrophysiologically abnormal infarct substrate; 2) the epicardial activation sequences during the VTs; 3) the locations of epicardial breakthrough sites; and 4) electrophysiologic evidence for activation of the Purkinje system and septum during the reentrant beats. Electrocardiographic imaging can noninvasively reconstruct electrophysiologic information on the epicardium during VT with intramural reentry, provide information about the location of the intramural components of reentry and image abnormal electrophysiologic substrates associated with infarction.
 Click to Enlarge
Click to Enlarge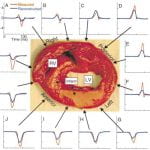 Click to Enlarge
Click to Enlarge
J.E. Burnes, B. Taccardi, R.S. MacLeod, Y. Rudy, “Noninvasive Electrocardiographic Imaging of Electrophysiologically Abnormal Substrate in Infarcted Hearts: A Model Study” Circulation 2000;101:533-540.
Myocardial infarction and subsequent remodeling create substrates with altered electrophysiological (EP) properties that are highly arrhythmogenic. Existing ECG methods cannot always detect the existence of such substrates nor provide any detailed information about their EP characteristics. A noninvasive method with such capabilities is greatly needed for identifying patients at risk of arrhythmias and for guidance and evaluation of therapy. Recently, we developed a noninvasive ECG imaging modality that can reconstruct epicardial EP information from body surface potentials. We extended its application to hearts with structural disease and examined its ability to detect and characterize abnormal EP substrates. Epicardial potentials were recorded with a 490-electrode sock from an open-chest dog. Recordings were obtained from a normal heart and from the same heart 2 hours after left anterior descending coronary artery occlusion and ethanol injection to create an infarct. Body surface potentials were generated from these epicardial potentials in a human torso model. Realistic geometry errors and measurement noise were added to the torso data, which were then used to noninvasively reconstruct epicardial potentials and electrograms (EGMs), with excellent accuracy. EP characteristics associated with the infarct substrate were reconstructed, including (1) a negative region over the infarct, (2) EGMs with large predominant negative deflections (eg, Q-wave EGMs), (3) Q-wave EGMs with superimposed RS deflections reflecting local activation of surviving myocardium within the infarct border zone, (4) reduced magnitudes of EGM negative derivatives, and (5) negative QRS integrals of EGMs over the infarct. ECG imaging can noninvasively detect and map abnormal EP substrates associated with infarction and structural heart disease.
J. E. Burnes, B. Taccardi, Y. Rudy,”A Noninvasive Imaging Modality for Cardiac Arrhythmias” Circulation 2000; 102:2152-2158.
The last decade witnessed an explosion of information regarding the genetic, molecular, and mechanistic basis of heart disease. Translating this information into clinical practice requires the development of novel functional imaging modalities for diagnosis, localization, and guided intervention. A noninvasive modality for imaging cardiac arrhythmias is not yet available. Present electrocardiographic methods cannot precisely localize a ventricular tachycardia (VT) or its key reentrant circuit components. Recently, we developed a noninvasive electrocardiographic imaging modality (ECGI) that can reconstruct epicardial electrophysiological information from body surface potentials. Here, we extend its application to image reentrant arrhythmias. Epicardial potentials were recorded during VT with a 490 electrode sock during an open chest procedure in 2 dogs with 4-day-old myocardial infarctions. Body surface potentials were generated from these epicardial potentials in a human torso model. Realistic geometry errors and measurement noise were added to the torso data, which were then used to noninvasively reconstruct epicardial isochrones, electrograms, and potentials with excellent accuracy. ECGI reconstructed the reentry pathway and its key components, including (1) the central common pathway, (2) the VT exit site, (3) lines of block, and (4) regions of slow and fast conduction. This allowed for detailed characterization of the reentrant circuit morphology.ECGI can noninvasively image arrhythmic activation on the epicardium during VT to identify and localize key components of the arrhythmogenic pathway that can be effective targets for antiarrhythmic intervention.
P. Jia, B. Punske, B. Taccardi, Y. Rudy , “Electrophysiologic Endocardial Mapping from a Noncontact Nonexpandable Catheter: A Validation Study of a Geometry-Based Concept” J Cardiovasc Electrophysiol 2000; 11:1238-1251.
The need for high resolution simultaneous mapping of cardiac excitation and arrhythmias on a beat-by-beat basis is widely recognized. Here we validate a noncontact mapping approach that combines a spiral catheter design with mathematical reconstruction to generate potential maps, electrograms, and activation maps (isochrones) on the entire left ventricular endocardial surface during a single beat. The approach is applicable to any heart chamber. The catheter is 3 mm (9 French) in diameter and carries 96 electrodes. Reconstruction accuracy is evaluated through direct comparison with endocardial data measured with 95 needle electrodes. Results show that endocardial potentials, electrograms, and isochrones are reconstructed with good accuracy during pacing from single or multiple sites (simulating ectopic activity). Pacing sites can be located to within 5 mm of their actual position, and intersite distances of 17 mm can be resolved during dual pacing. The reconstructed potential pattern reflects the intramural depth of pacing. The reconstructions are robust in the presence of geometric errors, and the accuracy is minimally reduced when only 62 catheter electrodes are used (32 are sufficient for pacing site localization).The study demonstrates that simultaneous endocardial mapping can be accomplished during a single beat from a spiral-shaped noncontact catheter with good accuracy.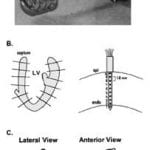 Click to Enlarge
Click to Enlarge
1997-1998
Y. Rudy, “ECGI: A Noninvasive Imaging Modality for Characterization of Intramural Myocardial Activation” J Electrocardiology 1999;32: 1-6.
H.S. Oster, B. Taccardi, R.L. Lux, P.R. Ershler, Y. Rudy, “Electrocardiographic Imaging: Noninvasive Characterization of Intramural Myocardial Activation from Inverse Reconstructed Epicardial Potentials and Electrograms” Circulation 1998; 97:1496-1507.
Y. Rudy, B. Taccardi, “Noninvasive Imaging and Catheter Imaging of Potentials, Electrograms and Isochrones on the Ventricular Surfaces” J Electrocardiology 1998; 30:19-23.
Z.W. Liu, P.Jia, L.A. Biblo, B. Taccardi, Y. Rudy, “Single-Beat Endocardial Mapping from a Noncontact Nonexpandable 9F Catheter: A Feasibility Study” Annals of Biomedical Engineering 1998; 26:994-1009.
H.S.Oster, B.Taccardi, R.L. Lux, P.R. Ershler, Y. Rudy, “Noninvasive Electrocardiographic Imaging: Reconstruction of Epicardial Potentials, Electrograms and Isochrones, and Localization of Single and Multiple Electrocardiac Events” Circulation 1997; 96: 1012-1024.
Z.W. Liu, P.Jia, P.R.Ershler, B.Taccardi, R.L. Lux, D.S. Khoury, Y. Rudy, “Noncontact Endocardial Mapping: Reconstruction of Electrograms and Isochrones from Intracavitary Probe Potentials” J Cardiovasc Electrophys 1997; 8: 415-431.
D.S. Khoury, B. Taccardi, R.L. Lux, P.R. Ershler, and Y. Rudy, “Reconstruction of Endocardial Potentials and Activation Sequences from Intracavitary Probe Measurements: Localization of Pacing Sites and Effects of Myocardial Structure” Circulation 1995; 91: 845-863.
B.J. Messinger-Rapport and Y. Rudy, “Non-Invasive Recovery of Epicardial Potentials in a Realistic Heart-Torso Geometry: Normal Sinus Rhythm” Circulation Research 1990; 66: 1023-1039.
Publication Years: 2005-2006 | 2002-2004 | 1997-1999
2005-2006
R.N. Ghanem, P. Jia, C. Ramanathan, K. Ryu, A. Markowitz, Y. Rudy, “Noninvasive Electrocardiographic Imaging (ECGI): Comparison to Intraoperative Mapping in Patients” Heart Rhythm Journal 2005; 2:339-354.
Cardiac arrhythmias are a leading cause of death and disability. Electrocardiographic imaging (ECGI) is a noninvasive imaging modality that reconstructs potentials, electrograms, and isochrones on the epicardial surface from body surface measurements. We previously demonstrated in animal experiments through comparison with simultaneously measured epicardial data the high accuracy of ECGI in imaging cardiac electrical events. Here, images obtained by noninvasive ECGI are compared to invasive direct epicardial mapping in open heart surgery patients. Three patients were studied during sinus rhythm and right ventricular endocardial and epicardial pacing (total of five datasets). Body surface potentials were acquired preoperatively or postoperatively using a 224-electrode vest. Heart-torso geometry was determined preoperatively using computed tomography. Intraoperative mapping was performed with two 100-electrode epicardial patches. Noninvasive potential maps captured epicardial breakthrough sites and reflected general activation and repolarization patterns, localized pacing sites to _1 cm and distinguished between epicardial and endocardial origin of activation. Noninvasively reconstructed electrogram morphologies correlated moderately with their invasive counterparts (cross correlation = 0.72 ± 0.25 [sinus rhythm], 0.67 ± 0.23 [endocardial pacing], 0.71 ± 0.21 [epicardial pacing]). Noninvasive isochrones captured the sites of earliest activation, areas of slow conduction, and the general excitation pattern. Despite limitations due to nonsimultaneous acquisition of the surgical and noninvasive data under different conditions, the study demonstrates that ECGI can capture important features of cardiac electrical excitation in humans noninvasively during a single beat. It also shows that general excitation patterns and electrogram morphologies are largely preserved in open chest conditions.
C. Ramanathan, R.N. Ghanem, P. Jia, K. Ryu, Y. Rudy, “Electrocardiographic Imaging (ECGI): A Noninvasive Imaging Modality for Cardiac Electrophysiology and Arrhythmia” Nature Medicine 2004; 10:422-428.
Over 7 million people worldwide die annually from erratic heart rhythms (cardiac arrhythmias), and many more are disabled. Yet there is no imaging modality to identify patients at risk, provide accurate diagnosis and guide therapy. Standard diagnostic techniques such as the electrocardiogram (ECG) provide only low-resolution projections of cardiac electrical activity on the body surface. Here we demonstrate the successful application in humans of a new imaging modality called electrocardiographic imaging (ECGI), which noninvasively images cardiac electrical activity in the heart. In ECGI, a multielectrode vest records 224 body-surface electrocardiograms; electrical potentials, electrograms and isochrones are then reconstructed on the heart’s surface using geometrical information from computed tomography (CT) and a mathematical algorithm. We provide examples of ECGI application during atrial and ventricular activation and ventricular repolarization in (i) normal heart (ii) heart with a conduction disorder (right bundle branch block) (iii) focal activation initiated by right or left ventricular pacing, and (iv) atrial flutter.
R.N. Ghanem, C. Ramanathan, P. Jia, Y. Rudy, “Heart-Surface Reconstruction and ECG Electrodes Localization Using Fluoroscopy, Epipolar Geometry and Stereovision: Application to noninvasive imaging of cardiac electrical activity“ IEEE Trans on Medical Imaging 2003;22:1307-1318
To date there is no imaging modality for cardiac arrhythmias which remain the leading cause of sudden death in the United States (300,000/yr.). Electrocardiographic imaging (ECGI), a noninvasive modality that images cardiac arrhythmias from body surface potentials, requires the geometrical relationship between the heart surface and the positions of body surface ECG electrodes. A photographic method was validated in a mannequin and used to determine the three-dimensional coordinates of body surface ECG electrodes to within 1 mm of their actual positions. Since fluoroscopy is available in the cardiac electrophysiology (EP) laboratory where diagnosis and treatment of cardiac arrhythmias is conducted, a fluoroscopic method to determine the heart surface geometry was developed based on projective geometry, epipolar geometry, point reconstruction, b-spline interpolation and visualization. Fluoroscopy-reconstructed hearts in a phantom and a human subject were validated using high-resolution computed tomography (CT) imaging. The mean absolute distance error for the fluoroscopy-reconstructed heart relative to the CT heart was 4 mm (phantom) and 10 mm (human). In the human, ECGI images of normal cardiac electrical activity on the fluoroscopy-reconstructed heart showed close correlation with those obtained on the CT heart. Results demonstrate the feasibility of this approach for clinical noninvasive imaging of cardiac arrhythmias in the interventional EP laboratory.
Y.H.He, R.N. Ghanem, A.L. Waldo, Y. Rudy, “An Interactive Graphical System for Automated Mapping and Display of Cardiac Rhythms” J Electrocardiology 1999;32:225-241.
Publication Years: 2009-Present | 2007-2008 | 2005-2006 | 2004
2009-Present
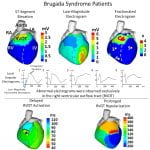
J. Zhang, F. Sacher, K. Hoffmayer, T. O’Hara, M. Strom, P. Cuculich, J. Silva, D. Cooper, M. Faddis, M. Hocini, M. Haïssaguerre, M. Scheinman, and Y. Rudy, “Cardiac Electrophysiologic Substrate Underlying the ECG Phenotype and Electrogram Abnormalities in Brugada Syndrome Patients”, Circulation. 2015. Vol 131:1950-1959.
The Brugada syndrome (BrS) is a highly arrhythmogenic cardiac disorder, but the electrophysiological (EP) substrate in the intact human heart remains ill-defined. The purpose of this study is to characterize the EP substrate in BrS patients using noninvasive ECG imaging (ECGI). The results indicate that the abnormal EP substrate is localized exclusively in the right ventricular outflow tract. And the findings reveal the co-existence of conduction and repolarization abnormalities in the BrS substrate. This study also compares the BrS EP substrate to right bundle branch block (generally considered benign), and demonstrates the capability of ECGI to differentiate between the two pathologies with similar ECGs.
 Click to Enlarge
Click to Enlarge
R. Vijayakumar, J.N. Silva, K.A. Desouza, R.L. Abraham, M. Strom, F. Sachar, G.F. Van Hare, M. Haïssaguerre, D.M. Roden, Y. Rudy, “Electrophysiologic Substrate in Congenital Long QT Syndrome: Noninvasive Mapping with Electrocardiographic Imaging (ECGI)”, Circulation. 2014. Vol 130:1936-1943.
This is the first comprehensive study in 25 patients of a hereditary cardiac arrhythmia using the novel ECGI technology developed in the Rudy laboratory. The Long QT syndrome is associated with fatal arrhythmias and sudden death and is caused by mutations in cardiac ion channels. The image above is an ECGI map of abnormal electrophysiological substrate in a patient with the Long QT syndrome.
S.B. Marrus, C.M. Andrews, D.H. Cooper, M.N. Faddis, and Y. Rudy, “Repolarization Changes Underlying Long-Term Cardiac Memory Due to Right Ventricular Pacing: Noninvasive Mapping With Electrocardiographic Imaging,” Circulation Arrhythmia and Electrophysiology. 2012. Vol 5 (4) pp. 773-781.
The aim of this work was to investigate the electrical changes which result from right ventricular pacing. It has been known for decades that prolonged RV pacing (or other abnormal activation patterns) result in repolarization changes which persist after cessation of the abnormal activation, a phenomenon termed “cardiac memory.” In this study, ECGI was used to reconstruct cardiac repolarization before, during, and after RV pacing. Two novel insights emerged from these results. First, the region close to the site of pacing exhibited local action potential prolongation resulting in a potentially arrhythmogenic dispersion of repolarization. Second, this dispersion of repolarization was only partially manifest during continuous RV pacing, raising the intriguing possibility that continuous pacing partially suppresses this electrical remodeling. Further work is needed to define the clinical implications of these observations but the results offer novel mechanistic insights into the sequelae of RV pacing.
P.S. Cuculich, Y. Wang, B.D. Lindsay, M.N. Faddis, R.B. Schuessler, R.J. Damiano, L. Li, and Y. Rudy, “Noninvasive Characterization of Epicardial Activation in Humans With Diverse Atrial Fibrillation Patterns,” Circulation. 2010 Oct.
The experiments in this paper first investigate the resolution of ECGI for identifying atrial ectopic sites, then use ECGI to map diverse patterns of atrial fibrillation noninvasively in human patients. The results of this work found that ECGI can determine atrial initiation sites with an accuracy of approximately 6 mm. Atrial fibrillation patterns observed in patients were very diverse featuring multiple wavelets, pulmonary vein and non-pulmonary vein focal sites, and rotor activity.
Y. Wang, P.S. Cuculich, J. Zhang, K.A. Desouza, R. Vijayakumar, J. Chen, M.N. Faddis, B.D. Lindsay, T.W. Smith, and Y. Rudy, “Noninvasive Electroanatomic Mapping of Human Ventricular Arrhythmias with Electrocardiographic Imaging”, Science Translational Medicine. 2011 Aug.
The majority of sudden cardiac death events are due to ventricular tachycardia (VT) . The ability to noninvasively image cardiac electrophysiology toward gaining insights about mechanisms of VT could strengthen our efforts to prevent VT, identify at-risk patients, and treat the arrhythmias more effectively. Noninvasive ECGI was performed on 26 patients with VT or symptomatic premature ventricle complexes. Among them, 23 patients participated in the catheter-based electrophysiology studies (EPS). Noninvasive ECGI images were compared to invasive findings at EPS with respect to local site of origin (SOO) of VT, myocardial depth of VT, and presumed mechanism of VT. ECGI identified the correct ventricle where VT originated in all cases (23 of 23). The ECGI SOO was consistent with invasive EPS SOO in 10 of 11 right ventricular sites and in 11 of 12 left ventricular sites. All focal (18 of 18) and reentrant (5 of 5) mechanisms determined from ECGI activation maps were in agreement with those determined by invasive EPS. Myocardial depth of the VT sources were accurately imaged by ECGI (5 of 5 epicardial VT, 7 of 8 non-epicardial VT) based on local electrogram characteristics. This paper reports the first findings from a noninvasive 3D electroanatomic mapping system, ECGI, in a series of patients undergoing catheter ablation for a broad range of VT types. The results suggest possible clinical usefulness for ECGI.
P.S. Cuculich, J. Zhang, Y. Wang, K.A. Desouza, R. Vijayakumar, P.K. Woodard, Y. Rudy. “The electrophysiological cardiac ventricular substrate in patients after myocardial infarction: noninvasive characterization with electrocardiographic imaging.” Journal of the American College of Cardiology. 2011 Oct.
The aim of this work was to noninvasively characterize the electrophysiological substrate of human ventricles after myocardial infarction. The results of this work reveal that epicardial electrograms in regions of scar are characterized by low amplitudes and fractionation. Criteria based on these characteristics were able to colocalize anatomical scar regions determined by MRI with a high degree of accuracy (sensitivity 89%, specificity 85%).
Y. Wang, R.B.Schuessler, R.J.Damiano, P.K. Woodard, Y. Rudy, “Noninvasive Electrocardiographic Imaging (ECGI) of Scar-Related Atypical Atrial Flutter”, Heart Rhythm Journal 2007 Dec.
Atrial arrhythmias, including atrial fibrillation, atrial flutter and other types of atrial tachycardia are common in humans. For instance, atrial fibrillation affects 0.5-1% of the general population, and more than 6% of people over 80 years old. Knowledge of electrophysiological properties of the underlying atrial substrate can improve diagnosis and treatment of these arrhythmias. In this case report, we describe the atrial substrate and arrhythmia mechanism of atypical atrial flutter in a patient who previously underwent catheter ablations for atrial fibrillation. ECGI detected and mapped regions of low voltages that coincided with scar tissue around the pulmonary veins (PVs) from the previous catheter ablation procedures, as verified during a Cox-Maze surgical procedure. The flutter reentry circuit was constrained by the scar and mainly confined to the left atrium (LA). The mitral isthmus between the coronary sinus and the PVs participated in the reentry circuit. ECGI also imaged a region of low potentials on the LAA, which is uncommon. Typically, the left atrial appendage (LAA) is a region of high voltage, as measured by endocardial mapping. During the Cox-Maze procedure, it was observed that the LAA was extensively scarred (from the underlying disease not from the prior ablation), consistent with the ECGI reconstruction of low voltages in this region.
Y. Wang, P.S. Cuculich, P.K. Woodard, B.D. Lindsay,Y. Rudy, “Focal Atrial Tachycardia after Pulmonary Vein Isolation: Noninvasive Mapping with Electrocardiographic Imaging (ECGI)”, Heart Rhythm Journal 2007; 4:1081-1084.
This report describes the first case in which ECGI was applied in a patient with a focal atrial tachycardia. In particular, ECGI accurately located the earliest site of activation in an atrium which had previously undergone two percutaneous pulmonary vein isolation procedures. The tachyarrhythmia was successfully terminated with RF ablation in the ECGI-determined location.
S Ghosh, JN Avari, EK Rhee, PK Woodard, Y Rudy. “Noninvasive Electrocardiographic Imaging (ECGI) of a Univentricular Heart with Wolff-Parkinson-White Syndrome.” Heart Rhythm. 2008;5:605-608.
This report describes for the first time, a case where ECGI was applied to a pediatric patient with a congenital structural heart defect. The patient was a 12 year old female who was diagnosed at birth with tricuspid and pulmonary atresia and interrupted inferior vena cava with azygous continuation to the superior vena cava. Her palliative surgeries included a Kawashima procedure followed by completion of her Fontan circulation with extracardiac hepatic diversion into the cavopulmonary circuit. Her 12-lead ECG showed persistent preexcitation consistent with Wolff-Parkinson-White syndrome and ECGI was used to localize the accessory pathway and guide intracardiac mapping during her electrophysiologic procedure. While the 12-lead ECG-based Arruda algorithm predicted a right lateral pathway, ECGI potential and activation maps showed pre-excitation in the anteroseptal area below the aortic valve annulus, which was further confirmed by invasive electroanatomic mapping (CARTO). This case study demonstrates the feasibility of ECGI in patients with complex congenital heart disease and engenders the promise of further application of the ECGI technology for understanding the underlying electrophysiology in such complicated and challenging cases.
S Ghosh, JN Avari, EK Rhee, PK Woodard, Y Rudy. “Noninvasive Electrocardiographic Imaging (ECGI) of epicardial activation before and after catheter ablation of the accessory pathway in a patient with Ebstein anomaly.” Heart Rhythm. 2008;5:857-860.
Ebstein anomaly is characterized by abnormal development of the tricuspid valve with the septal and often posterior leaflets of the valve displaced into the right ventricle. The abnormal development of the tricuspid valve often is associated with several conduction abnormalities including delayed intra-atrial conduction, right bundle branch block and ventricular pre-excitation. ECGI was used to image epicardial activation before and after ablation of accessory pathway in a patient with Ebstein anomaly. Her pre-ablation 12-lead ECG showed ventricular pre-excitation indicative of a posteroseptal epicardial pathway in the coronary sinus or its branches. ECGI activation map pre-ablation indicated a right posterolateral insertion which concurred with the findings of the electrophysiologic study and successful ablation. Post-ablation ECGI showed the earliest activation of RV delayed compared to the earliest activation of LV, indicating abnormalities in the right bundle branch conduction. The pre-ablation region of pre-excitation however, activated late post-ablation.
S Ghosh, EK Rhee, JN Avari, PK Woodard, Y Rudy. “Cardiac memory in patients with Wolff-Parkinson-White Syndrome: Noninvasive Imaging of Activation and Repolarization Before and After Catheter Ablation.” Circulation. 2008;118:907-915.
Cardiac memory refers to a change in ventricular repolarization induced by and persisting for minutes to months after cessation of a period of altered ventricular activation (eg, resulting from pacing or preexcitation in patients with Wolff-Parkinson-White syndrome). ECG imaging (ECGI) is a novel imaging modality for noninvasive electroanatomic mapping of epicardial activation and repolarization. Fourteen pediatric patients with Wolff-Parkinson-White syndrome and no other congenital disease, were imaged with ECGI a day before and 45 minutes, 1 week, and 1 month after successful catheter ablation. ECGI determined that preexcitation sites were consistent with sites of successful ablation in all cases to within a 1-hour arc of each atrioventricular annulus. In the preexcited rhythm, activation-recovery interval (ARI) was the longest (349±6 ms) in the area of preexcitation leading to high average base-to-apex ARI dispersion of 95±9 ms (normal is ![]() 40 ms). The ARI dispersion remained the same 45 minutes after ablation, although the activation sequence was restored to normal. ARI dispersion was still high (79±9 ms) 1 week later and returned to normal (45±6 ms) 1 month after ablation. The study demonstrates that ECGI can noninvasively localize ventricular insertion sites of accessory pathways to guide ablation and evaluate its outcome in pediatric patients with Wolff-Parkinson-White syndrome. Wolff-Parkinson-White is associated with high ARI dispersion in the preexcited rhythm that persists after ablation and gradually returns to normal over a period of 1 month, demonstrating the presence of cardiac memory. The 1-month time course is consistent with transcriptional reprogramming and remodeling of ion channels.
40 ms). The ARI dispersion remained the same 45 minutes after ablation, although the activation sequence was restored to normal. ARI dispersion was still high (79±9 ms) 1 week later and returned to normal (45±6 ms) 1 month after ablation. The study demonstrates that ECGI can noninvasively localize ventricular insertion sites of accessory pathways to guide ablation and evaluate its outcome in pediatric patients with Wolff-Parkinson-White syndrome. Wolff-Parkinson-White is associated with high ARI dispersion in the preexcited rhythm that persists after ablation and gradually returns to normal over a period of 1 month, demonstrating the presence of cardiac memory. The 1-month time course is consistent with transcriptional reprogramming and remodeling of ion channels.
C. Ramanathan, P. Jia, R.N. Ghanem, K. Ryu, Y. Rudy, “Activation and repolarization of the normal human heart under complete physiological conditions” Proc. Nat. Acad. Sci., U.S.A. (PNAS) 2006; 103:6309-14.
Knowledge of normal human cardiac excitation stems from isolated heart or intraoperative mapping studies under nonphysiological conditions. Here, we use a noninvasive imaging modality (electrocardiographic imaging) to study normal activation and repolarization in intact unanesthetized healthy adults under complete physiological conditions. Epicardial potentials, electrograms, and isochrones were noninvasively reconstructed. The normal electrophysiological sequence during activation and repolarization was imaged in seven healthy subjects (four males and three females). Electrocardiographic imaging depicted salient features of normal ventricular activation, including timing and location of the earliest right ventricular (RV) epicardial breakthrough in the anterior paraseptal region, subsequent RV and left ventricular (LV) breakthroughs, apex-to-base activation of posterior LV, and late activation of LV base or RV outflow tract. The repolarization sequence was unaffected by the activation sequence, supporting the hypothesis that in normal hearts, local action potential duration (APD) determines local repolarization time. Mean activation recovery interval (ARI), reflecting local APD, was in the typical human APD range (235 ms). Mean LV apex-to-base ARI dispersion was 42 ms. Average LV ARI exceeded RV ARI by 32 ms. Atrial images showed activation spreading from the sinus node to the rest of the atria, ending at the left atrial appendage. This study provides previously undescribed characterization of human cardiac activation and repolarization under normal physiological conditions. A common sequence of activation was identified, with interindividual differences in specific patterns. The repolarization sequence was determined by local repolarization properties rather than by the activation sequence, and significant dispersion of repolarization was observed between RV and LV and from apex to base.
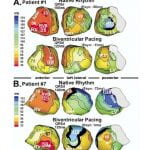
P. Jia, C. Ramanathan, R.N. Ghanem, K. Ryu, N. Varma, Y. Rudy, “Electrocardiographic Imaging of Cardiac Resynchronization Therapy in Heart Failure: Observations of Variable Electrophysiological Responses” Heart Rhythm Journal 2006;3:296-310.
Cardiac resynchronization therapy (CRT) for congestive heart failure patients with delayed left ventricular (LV) conduction is clinically beneficial in approximately 70% of patients. Unresolved issues include patient selection, lead placement, and efficacy of LV pacing alone. Being and electrical approach, detailed electrical information during CRT is critical to resolving these issues. However, electrical data from patients have been limited because of the requirement for invasive mapping. The purpose of this study was to provide observations and insights on the variable electrophysiologic responses of the heart to CRT using electrocardiographic imaging (ECGI). ECGI was conducted in eight patients undergoing CRT during native rhythm and various pacing modes. In native rhythm (six patients), ventricular activation was heterogeneous, with latest activation in the lateral LV base in three patients and in the anterolateral, midlateral, or inferior LV in the remainder of patients. Anterior LV was susceptible to block and slow conduction. Right ventricular pacing improved electrical synchrony in two of six patients. LV pacing in three of four patients involved fusion with intrinsic excitation resulting in electrical resynchronization similar to biventricular pacing. Although generally electrical synchrony improved significantly with biventricular pacing, it was not always accompanied by clinical benefit. Results suggest that (1) when accompanied by fusion, LV pacing alone can be as effective as biventricular pacing for electrical resynchronization; (2) right ventricular pacing is not effective for resynchronization; and (3) efficacy of CRT depends strongly on the patient-specific electrophysiologic substrate.
A. Intini, R.N. Goldstein, P. Jia, C. Ramanathan, K. Ryu, B. Giannattasio, R. Gilkeson, B.S. Stambler, P. Brugada, W.G. Stevenson, Y. Rudy, A.L. Waldo, “Electrocardiographic Imaging (ECGI), a Novel Diagnostic Modality used for Mapping of Focal Left Ventricular Tachycardia in a Young Athlete” Heart Rhythm Journal 2005;2:1250-1252
We report the first clinical application of electrocardiographic imaging (ECGI), a new, noninvasive imaging modality for arrhythmias, in an athlete with focal ventricular tachycardia (VT) originating from a left ventricular (LV) diverticulum. A reconstructed map of the epicardial activation sequence during a single premature ventricular complex (PVC) of an identical QRS morphology to the clinical VT, generated from 224-electrode body surface ECGs and a chest CT (ECGI), localized the PVC to the site of the diverticulum. This correlated with subsequent maps obtained using standard techniques. We describe the first case that used ECGI to guide diagnosis and therapy of a clinical tachyarrhythmia.
C. Ramanathan, R.N. Ghanem, P. Jia, K. Ryu, Y. Rudy, “Electrocardiographic Imaging (ECGI): A Noninvasive Imaging Modality for Cardiac Electrophysiology and Arrhythmia” Nature Medicine 2004; 10:422-428.
Over 7 million people worldwide die annually from erratic heart rhythms (cardiac arrhythmias), and many more are disabled. Yet there is no imaging modality to identify patients at risk, provide accurate diagnosis and guide therapy. Standard diagnostic techniques such as the electrocardiogram (ECG) provide only low-resolution projections of cardiac electrical activity on the body surface. Here we demonstrate the successful application in humans of a new imaging modality called electrocardiographic imaging (ECGI), which noninvasively images cardiac electrical activity in the heart. In ECGI, a multielectrode vest records 224 body-surface electrocardiograms; electrical potentials, electrograms and isochrones are then reconstructed on the heart’s surface using geometrical information from computed tomography (CT) and a mathematical algorithm. We provide examples of ECGI application during atrial and ventricular activation and ventricular repolarization in (i) normal heart (ii) heart with a conduction disorder (right bundle branch block) (iii) focal activation initiated by right or left ventricular pacing, and (iv) atrial flutter.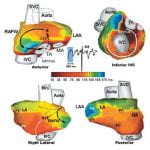
Click to Enlarge
Publication Years: 1993-1996 | 1989-1992 | 1978-1988 1993-1996 J .Ortiz, S. Niwano, H. Abe, H.X. Gonzalez, Y. Rudy, N. Johnson and A.L. Waldo, “Mapping the Conversion of Atrial Flutter to Atrial Fibrillation and Atrial Fibrillation to Atrial Flutter – Insights into Mechanism” Circulation Research 1994; 74: 882-894. J. Liebman, C.W. Thomas, and Y. Rudy, “Electrocardiographic Body Surface Potential Mapping Many Years After Successful Surgery for Coarctation of the Aorta” J Electrocardiology 1993; 26: 25-41.
1989-1992 J. Liebman, B. Olshansky, A. Zeno, A. Geha, Y. Rudy, W. Henthorn, M. Cohen, and A.L. Waldo, “Electrocardiographic Body Surface Potential Mapping in the Wolff – Parkinson -White Syndrome: Non-invasive Determination of the Ventricular Insertion Sites of AV Connections” Circulation 1991; 83: 886-901. Y. Reich, C.W. Thomas, J. Liebman, Y.H. Pao, and Y. Rudy, “Multi-Category Classification of Body Surface Potential Maps” IEEE Trans Biomed Eng BME 1990; 37: 945-955. J. Liebman, C.W. Thomas, R. Fraenkel, and Y. Rudy, “Analysis of the Hypoplastic Right Ventricle Utilizing Electrocardiographic Body Surface Potential Mapping” J Electrocardiology 1989; 22: 195-209. D. Khoury, H. McAlister, B. Wilkoff, T. Simmons, Y. Rudy, R. McCowan, V. Morant, L. Castle, and J. Maloney, “Continuous Right Ventricular Volume Assessment by Catheter Measurement of Impedance for Antitachycardia System Control” PACE 1989; 12: 1918-1926.
1978-1988 J.N. Amoore, Y. Rudy, and J. Liebman, “Respiration and the ECG. A Study Using Body Surface Potential Maps” J Electrocardiology 1988; 21: 263-271. L. Widman, J. Liebman, C.W. Thomas, R. Fraenkel, and Y. Rudy, “Electrocardiographic Body Surface Potential Maps of the QRS and T of Normal Young Adults – Qualitative Description and Selected Quantifications” J Electrocardiology 1988; 21: 121-136. J. Liebman, Y. Rudy, P. Diaz, C.W. Thomas, and R. Plonsey, “The Spectrum of Right Bundle Branch Block as Manifested in Electrocardiographic Body Surface Potential Maps” J Electrocardiology 1984; 17: 329-346. Y. Rudy, R. Wood, R. Plonsey, and J. Liebman, “The Effect of High Lung Conductivity on ECG Potentials: Results Obtained from Human Subjects Undergoing Bronchopulmonary Lavage” Circulation 1982; 65: 440-445. J. Liebman, C. Thomas, Y. Rudy , and R. Plonsey, “Electrocardiographic Body Surface Potential Maps of the QRS of Normal Children” J Electrocardiology 1981; 14: 249-260.
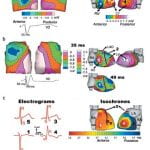 Click to Enlarge
Click to Enlarge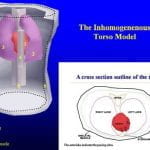 Click to Enlarge
Click to Enlarge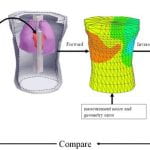 Click to Enlarge
Click to Enlarge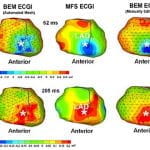 Click to Enlarge
Click to Enlarge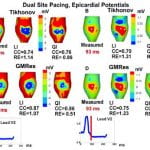 Click to Enlarge
Click to Enlarge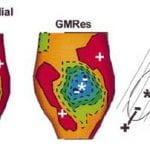 Click to Enlarge
Click to Enlarge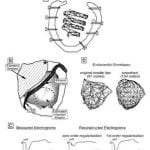 Click to Enlarge
Click to Enlarge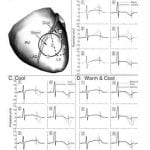 Click to Enlarge
Click to Enlarge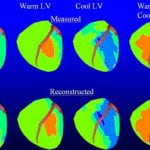 Click to Enlarge
Click to Enlarge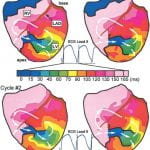 Click to Enlarge
Click to Enlarge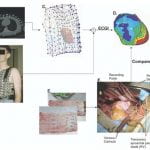 Click to Enlarge
Click to Enlarge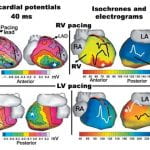 Click to Enlarge
Click to Enlarge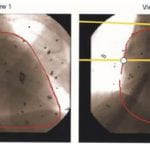 Click to Enlarge
Click to Enlarge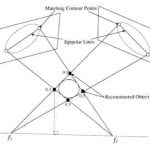 Click to Enlarge
Click to Enlarge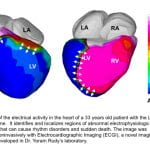 Click to Enlarge
Click to Enlarge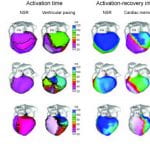 Click to Enlarge
Click to Enlarge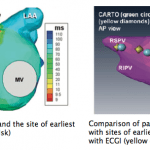 Click to Enlarge
Click to Enlarge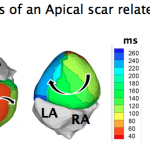 Click to Enlarge
Click to Enlarge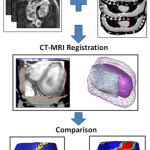 Click to Enlarge
Click to Enlarge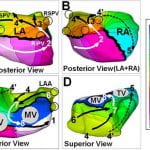 Click to Enlarge
Click to Enlarge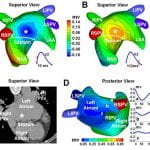 Click to Enlarge
Click to Enlarge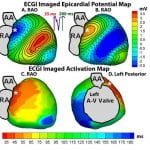 Click to Enlarge
Click to Enlarge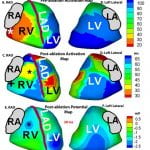 Click to Enlarge
Click to Enlarge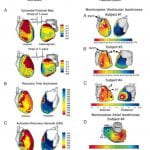
 Click to Enlarge
Click to Enlarge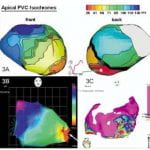 Click to Enlarge
Click to Enlarge