Models and Simulations > Ion Channels | Cardiac Cells | Cardiac Tissue
Kleber, A. G. and Y. Rudy (2004). “Basic mechanisms of cardiac impulse propagation and associated arrhythmias.” Physiol Rev 84(2): 431-88.
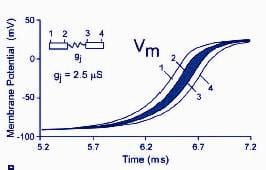
By connecting our cell models to form various tissue configurations we have performed studies revealing basic mechanisms of action potential conduction in cardiac tissue. In several key publications we investigated the role of intercellular coupling through gap junctions on propagation and repolarization of the action potential. We demonstrated the importance of ICa(L) during discontinuous conduction, when cells are poorly coupled.
Recently, we have focused attention on simulation of tissue representing the 5-day old canine infarct epicardial border zone (EBZ) which serves as a paradigm for electrical remodeling following an insult to the myocardium. Almost all studies in this model so far have focused on altered conduction properties, with much less attention given to altered repolarization. To help fill this gap, we will characterize the properties of rate-dependent repolarization in this model, and will study repolarization based mechanisms of arrhythmias.
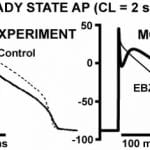
The remodeled EBZ AP is simulated by incorporating the measured alterations of ion channel densities and kinetics; in agreement with experiment its morphology is triangularized, it lacks a phase-1 notch, and it shows reduced excitability and prolonged post-repolarization refractoriness. Importantly, its capacity for APD rate-adaptation and restitution is greatly reduced at fast rates typical of tachyarrhythmias. All of these properties are important factors in the initiation and sustenance of arrhythmias and should be represented in models used for their studies. The control and EBZ cell models we developed represent these properties and provide the cellular elements for the studies of arrhythmias in the multicellular EBZ and its surrounding tissue.
Propagation of excitation in the heart involves action potential (AP) generation by cardiac cells and its propagation in the multicellular tissue. AP conduction is the outcome of complex interactions between cellular electrical activity, electrical cell-to-cell communication, and the cardiac tissue structure. As shown in this review, strong interactions occur among these determinants of electrical impulse propagation. This review attempts to synthesize results from computer simulations and experimental preparations to define mechanisms and biophysical principles that govern normal and abnormal conduction in the heart.