Background | Theory | Computational Implementation | Experimental Validation
Experimental Validation
Prior to Application in human subjects, ECGI was thoroughly tested and validated in experimental preparations. Of particular value were the experiments in a torso-tank that was designed to enable simultaneous measurement of torso potentials (VT) and epicardial potentials (VE). In this scheme,VT provided the input data for ECGI while measured VEprovided a gold standard for evaluating ECGI accuracy.
 The torso-tank experimental setup, developed by Bruno Taccardi and co-workers at the Universities of Parma (Italy) and Utah, constitutes an optimal tool that we have used for detailed and rigorous evaluation of the ECGI methodology. This experimental setup consists of a tank in the shape of a 10-year-old boy’s torso filled with an electrolytic solution, with a perfused dog heart (connected to the circulation of a support dog) suspended in the correct anatomical position within the torso volume. The torso tank system included 384 torso surface electrodes and 384 rods projecting radially from the torso surface toward the center of the tank. Each rod had an electrode at its tip and multiple electrodes along its length within the torso volume, for a total of 918 electrodes. The rods at the level of the heart were pushed towards the epicardium so that the tip electrodes created an epicardial envelope about 1cm from the epicardial surface.
The torso-tank experimental setup, developed by Bruno Taccardi and co-workers at the Universities of Parma (Italy) and Utah, constitutes an optimal tool that we have used for detailed and rigorous evaluation of the ECGI methodology. This experimental setup consists of a tank in the shape of a 10-year-old boy’s torso filled with an electrolytic solution, with a perfused dog heart (connected to the circulation of a support dog) suspended in the correct anatomical position within the torso volume. The torso tank system included 384 torso surface electrodes and 384 rods projecting radially from the torso surface toward the center of the tank. Each rod had an electrode at its tip and multiple electrodes along its length within the torso volume, for a total of 918 electrodes. The rods at the level of the heart were pushed towards the epicardium so that the tip electrodes created an epicardial envelope about 1cm from the epicardial surface.
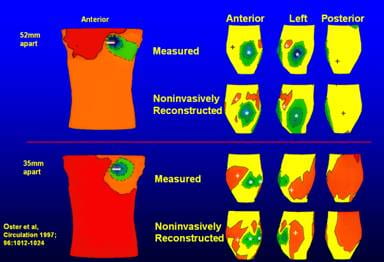
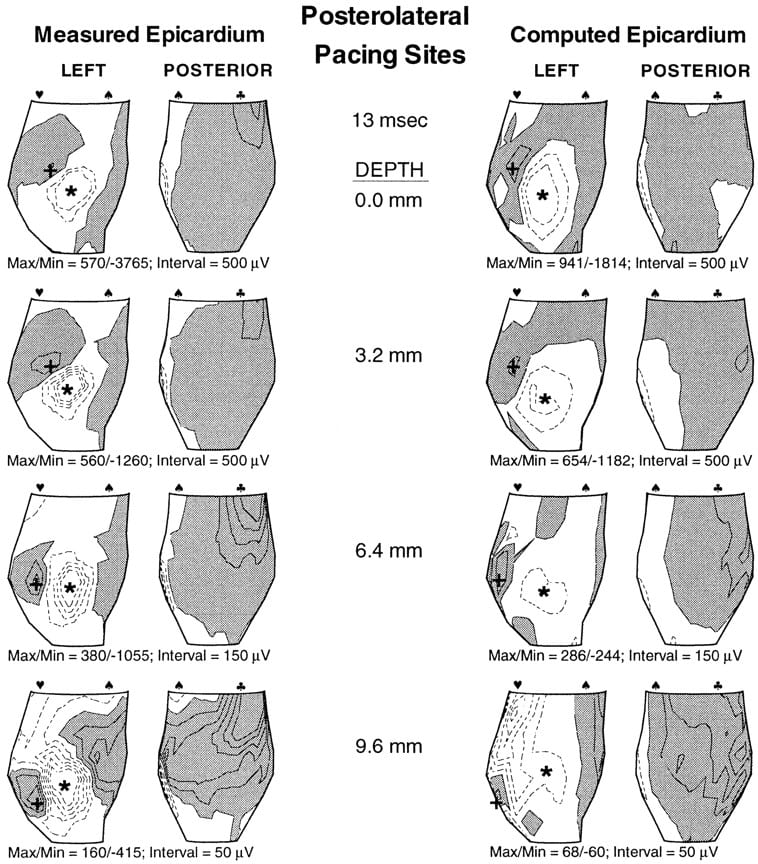
In one set of experiments, local electrocardiac events were initiated by pacing the dog heart from various epicardial sites. Body surface potential measurements (384 electrodes) were used to compute epicardial potentials noninvasively. The accuracy of reconstructed epicardial potentials was evaluated by direct comparison to measured ones (134 electrodes). Protocols included pacing from single sites and simultaneously from two sites with various intersite distances. Body surface potentials showed a single minimum for both single and double site pacing (intersite distances of 52, 35 and 17mm).
Oster HS1, Taccardi B, Lux RL, Ershler PR, Rudy Y. Electrocardiographic imaging: Noninvasive characterization of intramural myocardial activation from inverse-reconstructed epicardial potentials and electrograms. Circulation. 1998 Apr 21;97(15):1496-507.
Noninvasively reconstructed epicardial electrograms, potentials and isochrones closely approximated the measured ones. Single pacing sites were reconstructed to within 10mm of their measured positions. Dual sites were located accurately and resolved for the above intersite distances. Regions of sparse and crowded isochrones, indicating spatial nonuniformities of epicardial activation spread, were also reconstructed by ECGI.
Oster HS1, Taccardi B, Lux RL, Ershler PR, Rudy Y. Electrocardiographic imaging: Noninvasive characterization of intramural myocardial activation from inverse-reconstructed epicardial potentials and electrograms. Circulation. 1998 Apr 21;97(15):1496-507.
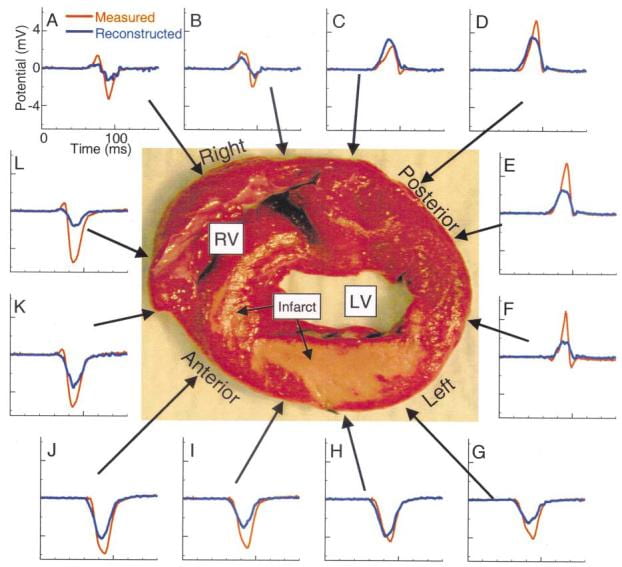
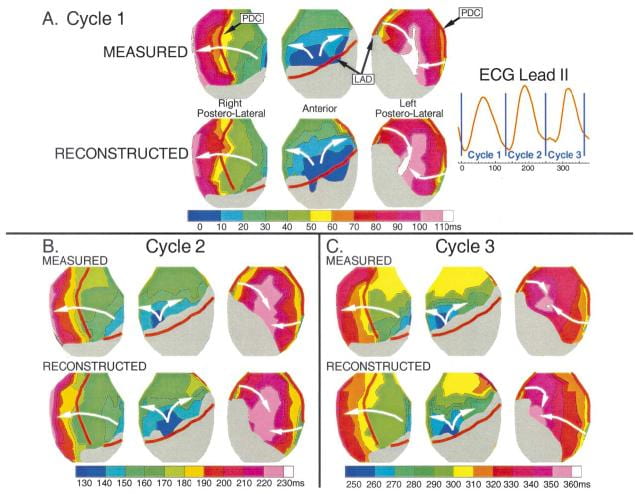
In another study, we evaluated ECGI’s ability to map intramural reentrant ventricular tachycardia (VT). A dog heart with a four-day-old infarct was suspended in the human shaped torso-tank. ECGI reconstructions were performed during right atrial pacing and nine cycles of VT.
 Burnes JE1, Taccardi B, Ershler PR, Rudy Y. Noninvasive electrocardiogram imaging of substrate and intramural ventricular tachycardia in infarcted hearts. J Am Coll Cardiol. 2001 Dec;38(7):2071-8.
Burnes JE1, Taccardi B, Ershler PR, Rudy Y. Noninvasive electrocardiogram imaging of substrate and intramural ventricular tachycardia in infarcted hearts. J Am Coll Cardiol. 2001 Dec;38(7):2071-8.
Noninvasively reconstructed potential maps, electrograms and isochrones identified: 1) the location of electrophysiologically abnormal infarct substrate; 2) the epicardial activation sequences during the VT cycles; 3) the locations of epicardial breakthrough sites; and provided 4) electrophysiologic evidence for activation of the Purkinje system and septum during the reentrant beats.
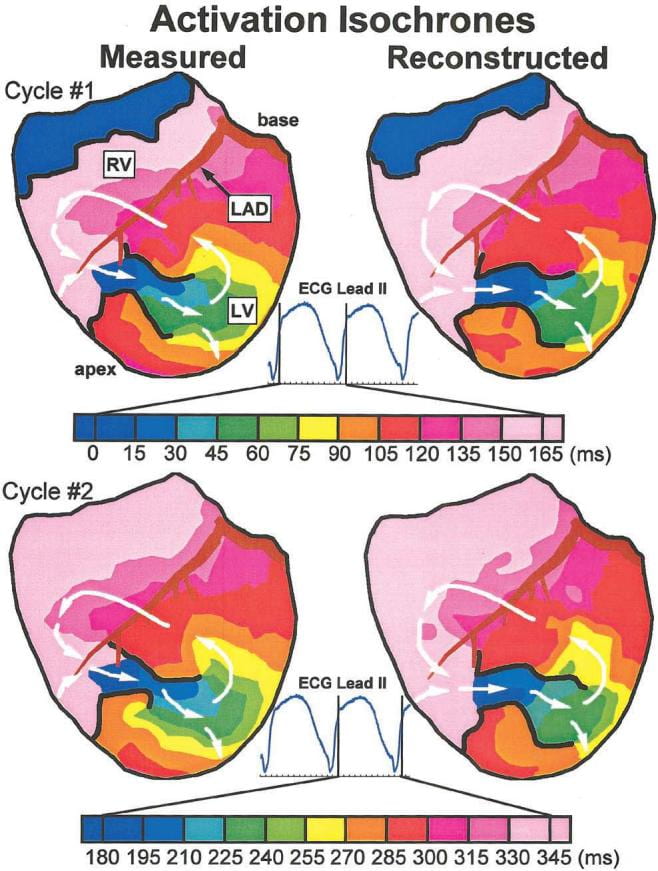
Another study was designed to evaluate ECGI ability to image details of an epicardial reentry circuit during VT.
Burnes JE, Taccardi B, Rudy Y. A noninvasive imaging modality for cardiac arrhythmias. Circulation. 2000 Oct 24;102(17):2152-8.
Epicardial potentials were recorded during VT with a 490 electrode sock from two open chest dogs with 4-day-old myocardial infarctions. Body surface potentials were generated from these epicardial potentials in a human torso model (a “virtual torso-tank”). Realistic geometry errors and measurement noise were added to the torso data, which were then used to noninvasively reconstruct epicardial isochrones, electrograms, and potentials with excellent accuracy. ECGI reconstructed the reentry pathway and its key components, including (1) the central common pathway, (2) the VT exit site, (3) lines of block, and (4) regions of slow and fast conduction. This allowed for detailed characterization of the reentrant circuit morphology.
These studies show that ECGI can noninvasively image arrhythmic activation on the epicardium during VT to identify and localize key components of the arrhythmogenic pathway that can be effective targets for antiarrhythmic intervention.
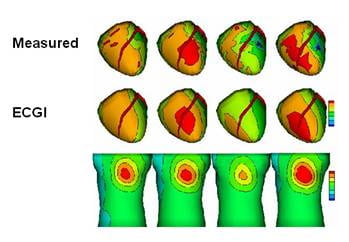
Studies were also conducted to demonstrate the feasibility of noninvasively reconstructing regional repolarization properties on the epicardial surface of the heart with ECGI. Given the important role of repolarization abnormalities in arrhythmogenesis, the ability to image substrates with abnormal repolarization noninvasively is of clinical significance, especially in view of the limitations of methods that rely on body-surface ECG data alone (e.g., QT dispersion).
Ghanem RN, Burnes JE, Waldo AL, Rudy Y. Imaging dispersion of myocardial repolarization, II: noninvasive reconstruction of epicardial measures. Circulation. 2001 Sep 11;104(11):1306-12.
ECGI imaging of repolarization could be used for risk stratification, for more specific diagnosis of repolarization abnormalities, and for monitoring the effects of therapeutic interventions (eg, repolarization-modifying antiarrhythmic drugs).